Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.101 no.8 Madrid ago. 2009
Treatment of irritable bowel syndrome with probiotics. An etiopathogenic approach at last?
Tratamiento con probióticos del síndrome de intestino irritable. ¿Por fin un enfoque etiopatogénico?
M. Bixquert Jiménez
Department of Medicine. School of Medicine (Gastroenterology Teaching Unit). University of Valencia. Spain. Department of Digestive Medicine. Hospital Arnau de Vilanova. Valencia, Spain
ABSTRACT
Irritable bowel syndrome (IBS) is the most common functional digestive disorder, and may affect 11-20% of the adult population in industrialized countries. In accordance with Rome III criteria (2006) IBS involves abdominal pain and bowel habit disturbance, which are not explained by structural or biochemical abnormalities. Several hypotheses attempt to account for the pathophysiology of IBS, but the etiology still remains uncertain or obscure, perhaps multifactorial. Abnormalities in colonic microflora have recently been suggested in such patients, as has abnormal small-intestine bacterial overgrowth (SIBO), or in particular a significant reduction in the amount of intraluminal Bifidobacteria or Lactobacilli, with consequences like the production of colonic gas, and motility or sensitivity disturbances of the intestinal tract. The disorder is difficult to treat, and the wide spectrum of non-drug and drug treatments shows our ignorance about the cause of the condition. Newer drugs, both pro- and anti-serotonin, have failed to show long-term efficacy or have been withdrawn due to concerns about harmful effects.
Recent research has provided increasing support for the idea that disturbances of intestinal microbiota occur in patients with IBS, and that such abnormalities may contribute to IBS symptoms. Studies in Scandinavian countries in the last ten years emphasize the role of probiotics in the modulation of intestinal microbiota, and as a consequence in the regulation of the motility and hypersensitivity of the digestive tract. Although results between studies are difficult to compare because of differences in study design, probiotic dose, strain, and duration of therapy, some studies show symptom improvement. Lactobacilli are found among the normal bacterial flora of the gastrointestinal tract, and Lactobacillus plantarum (Lp) is one of the species frequently isolated from the human mucosa, which is capable of surviving the low pH of the stomach and duodenum, resisting the effect of bile acids in the upper small intestine when ingested, and temporarily colonizing the gastrointestinal tract by binding to the intestinal and colonic mucosa. Concurrent with colonization by Lp there is a decrease in bacterial groups with gas-producing ability, such as Veillonella spp. and Clostridia spp. Evidence has now accumulated to suggest the efficacy of certain probiotics like Lp299v, which may be capable of bringing about a significant reduction in pain, abdominal distension and flatulence, while increasing health-related quality of life in IBS.
Key words: Functional digestive disorders. Irritable bowel syndrome (IBS). Intestinal microflora. Mucosal associated microbiota (MAM).
Introduction: irritable bowel syndrome, the paradigm of functional digestive disorders
Irritable bowel syndrome (IBS) is characterized by abdominal pain or discomfort, which is relieved by defecation or the passage of gas, and is associated with changes in stool frequency and/or consistency, without physical, radiological or endoscopic abnormalities or laboratory findings indicating organic disease. Sometimes there is a sensation of incomplete defecation, burning pain on defecation, urgency to defecate, rectal tenesmus, and mucorrhea. According to Rome III (1) there are several types of IBS: a) diarrhea-predominant IBS; b) constipation-predominant IBS; c) alternating IBS (sometimes diarrhea, sometimes constipation); and d) undefined IBS. It affects the quality of life of those who suffer from it, and accounts for the use of large amounts of healthcare resources, both in Primary and Specialist Care (2).
Other gastrointestinal symptoms are also common: esophageal balloon, heartburn, chest pain, early satiety, abdominal bloating or distension, and flatulence; and extragastrointestinal symptoms: asthenia, adynamia, headache, dizziness, sleep disorders, pollakiuria, neck pain, back pain, dysmenorrhea, dyspareunia, fibromyalgia. More than 50% have at least one somatic comorbid condition with gastrointestinal discomfort, and in those who have several conditions abdominal symptoms are more serious; they have more psychosocial or mental complaints, and they are absent from work due to acute common diseases. This comorbidity has a significant influence on the patient's consultation pattern, clinical and diagnostic management, and therapeutic plan (3).
IBS has a prevalence of 12-20% of the population worldwide and 11-14% in Spain; 30-40% consult their doctor, accounting for 12% of visits to general practitioners, 28% of visits to specialists, and a significant percentage of visits to outpatient departments of tertiary level hospitals. It is 2-3 times more common in women, especially in Primary Care. Although the symptoms of IBS are used to establish the suspected diagnosis, individually they are not sufficiently sensitive or specific (4). Predictive value increases when age below 50 and an absence of warning symptoms or signs are considered, which means that IBS can be suspected with a sensitivity of 96% and a specificity of 72%, which is not the case with another common functional digestive disorder, functional dyspepsia (5). Abdominal bloating or distension affects 15-30% of the general population, but 75-90% in IBS (especially in women and if there is constipation). In all, 28% of these patients suffer from it all the time, which is slightly less than abdominal pain (33%). Those seen in specialist care and at tertiary centers complain particularly of this, stating that such discomfort is greater than that of abdominal pain itself in 72% of cases. It is worse in the afternoon and evening, but better at night; it becomes worse when standing and is relieved when in a supine position. There is no correlation with defecation or retaining flatus, although it is worsened by eating and menstruation (6).
The etiopathogenesis, which is not fully understood, may be multifactorial (Table I), as is the pathophysiology, which is attributed to alterations in gastrointestinal motility, visceral hypersensitivity, dysfunction of the brain-gut axis or certain psychosocial factors (Table II). It has been suggested that there may be some autonomic dysfunction with an increased or sustained response to normal psychophysical stress; on measuring salivary chromogranin A, a derivative of catecholamines that is released in response to acute stress, it is found to be high in patients with IBS and may be reduced using muscle relaxation techniques (7). Although genetic factors play a minor role compared to learned behavior (8), they do influence symptomatic expression and especially therapeutic response (9). The management of intestinal gas is different in IBS patients and control subjects; whilst the latter expel the gas infused into the jejunum rapidly, the former retain it, causing them symptoms (10), regardless of whether or not intestinal gas production is increased.
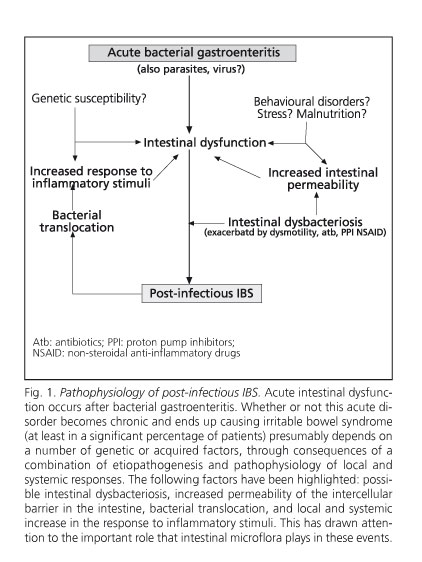
Despite being considered a non-organic syndrome, it has certain organic components, as is the case with post-infectious and inflammatory IBS (11), which are presented in figure 1. Although post-infectious IBS explains only 23-35% of IBS cases, it shows the relationship between exposure to the infectious agent Æ mucosal (and perhaps partly systemic) inflammation Æ clinical expression of IBS. It would be better described as an "organic" disorder in its pathogenesis, or at least "dysfunctional" (12,13). In 55 IBS patients increased levels of peripheral cytokines TNFα, IL1β, and IL6 were found at baseline in diarrhea-predominant IBS, and IL1β after administering E. coli LPS in both diarrhea-predominant IBS and constipation-predominant IBS, showing that TNFα elevation correlates with anxiety levels (13). Although little is known about intestinal hormone secretion in IBS, high levels of CCK and VIP were found in the plasma and rectosigmoid mucosa, whilst substance P and somatostatin were normal. However, neuropeptide Y levels in the plasma and rectosigmoid mucosa are lower in diarrhea-predominant IBS, but not in constipation-predominant IBS (14). Thirty-eight patients with normal colonic histology using conventional techniques, out of 77 IBS patients, showed an increase in intraepithelial lymphocytes and CD3 and CD25 cells in the lamina propria, suggesting immune activation; these abnormalities were even more evident in 31 additional patients who showed microscopic inflammation (15).
There is colorectal hypersensitivity in patients with IBS, especially in diarrhea-predominant IBS, expressed by: a) increased ileocolonic response to bile acid perfusion; b) reduced rectal adaptation to distension; and c) reduced pain threshold to rectal distension. Hypervigilance to digestive events would contribute to this, perhaps due to inadequate processing of afferent information in certain areas of the CNS, such as the anterior cingulated cortex, the amygdala, and the dorsomedial frontal cortex, as shown by dynamic MRI studies, which indicate an increase in the vascularization of these areas in response to colon stimulation that does not occur in controls or patients with ulcerative colitis (16) and which is reversed by pre-treatment with amitriptyline (17). IBS patients who consult their doctor are more prone to surgical interventions such as appendicectomy, cholecystectomy and hysterectomy; this is because symptoms can be mistaken in these prevalent conditions. It was recently pointed out that patients who have undergone cholecystectomy with follow-up as a cohort for 10-15 years have twice as high a risk of suffering from IBS, especially the diarrhea-predominant form (three times as high as constipation-predominant IBS). It is assumed that the continued presence of bile salts in the colon may give rise to symptoms of IBS (18), as these patients are known to have a hyperresponse to these substances.
Diagnosis is fundamentally clinical, and is based on the Rome III criteria of 2006 (1) (Table III), with the isolated exclusion of metabolic or organic diseases (benign or malignant) according to the patient's personal or family history. In any of the types there may be psychological, psychosocial or psychiatric alterations, or an exaggerated intestinal response to everyday stress. If there is abdominal bloating or distension, it is advisable to rule out lactose, fructose or trehalose intolerance, an excessive intake of insoluble fiber, and small intestinal bacterial overgrowth.
Treatment of IBS has been based on the predominant symptom. It must be individualized, emphasizing the absence of a serious or worrying condition, and straightforwardly explaining the nature and cause of the symptoms. But the measures available at present (spasmolytics or antidepressants at low doses for the pain, anti-diarrhea agents or 5HT3 antagonists for diarrhea, lubiprostone, bulk-forming laxatives or 5HT4 agonists for constipation, etc.) have only proven slightly more effective in comparison with placebo (which in IBS is 35-45%), and none have been capable of altering its natural course, as the effect disappears as soon as treatment is stopped. Patients often resort to alternatives such as medicinal herbs, homeopathy, acupuncture, hypnosis or psychotherapy, but randomized controlled studies with these types of therapy are non-existent or are compromised by their low methodological quality, and in general such treatments are not recommended. As regards acupuncture, the 2006 Cochrane review showed that the effect of real acupuncture compared with "sham acupuncture" was no more effective on any symptom of IBS or on the subjective perception of general well-being (19). On many occasions the findings of certain authors have not been confirmed by others (20-24). Alosetron or cilansetron may cause ischemic colitis, and tegaserod may cause severe cardiovascular or cerebrovascular events, which means that the use of serotonin receptor modulators is subject to a comprehensive management programme for potential risk in the USA, and is not considered in Europe (25).
IBS patients are known to self-medicate very often. A review of the use of OTC drugs and medicines in the context of primary care (3) shows that they take antacids (x 8), PPIs (x 3), fiber and laxatives (x 10), antidiarrhea agents (x 50), antispasmodics (x 30), antidepressants (x 3), sedative-hypnotics (x 2), and analgesics (x 2) more frequently than controls.
There is now a tendency to return to an etiopathogenesis- or pathophysiology-based therapeutic approach, attempting to influence the possible existence of intestinal dysbacteriosis, altered intestinal fermentation, excess production or alteration in the management of intestinal gas, but also subclinical mucosal inflammation, especially in patients in whom the onset of IBS followed an episode of acute bacterial gastroenteritis, abdominal resection surgery, or antibiotics, antineoplastic drugs or immunosuppressants, because, as is well known, these can all affect the normal balance of intestinal microflora (26,27). King et al. (28) found that colonic gas production was higher in patients with IBS than in controls, and that this increase and its symptoms could be normalized with diets that excluded insoluble fiber. Even Nobaek et al. (29), after administering Lactobacillus plantarum (Lp 299v) or placebo in an oatmeal soup to 60 IBS patients for 4 weeks, showed: a) the presence of Lp in rectal biopsies; b) that flatulence was reduced by 52% in the active group (25% in the placebo); and c) that abdominal pain was reduced in both groups (35% in those who received Lp; 22% in the placebo). Improvement in the group to which Lp was administered remained for 12-months.
Thus, one therapeutic approach in IBS could be to modulate intestinal microflora to correct an imbalance, because at present IBS still represents a significant therapeutic challenge in that only 15-20% of patients are satisfied with the medium- to long-term result of the treatment that they receive (30). This is what was said over 12 years ago: "...we suggest that probiotics (specifically Lactobacillus spp., Streptococcus thermophylus and Bifidobacterium spp.) stabilize the intestinal microflora, modulate hypersensitivity reactions, and promote intestinal barrier mechanisms, but these properties should be proven in carefully planned and controlled studies in humans." (31).
Pathophysiological basis for the use of probiotics in the treatment of IBS
Physiological anatomy of the intestinal mucosa
The mucosa of the gut is a single-cell layer consisting of four types of cells: enterocytes, mucus-secreting goblet cells, neuroendocrine cells, and membrane or M cells, which belong to the immune system and are particularly abundant in the terminal ileum and surrounding the lymph nodes; they bind to macromolecules or pathogens, which they process and transport to the mucosa-associated lymphoid tissue (MALT). There are also other defensive and immune cells that come from the peripheral circulation: monocytes, T and B lymphocytes, neutrophils, eosinophils, macrophages, plasmocytes, and dendritic or Cajal cells; the relationships between intestinal microflora, mucosa, and immune response can be seen in figure 2. The first-line intestinal defense mechanism is the local secretion of IgA by plasma cells, which is combined with intraluminal antigens to prevent absorption. If the antigen attack passes this barrier then an IgM- and IgG-based systemic immune response occurs (32).
The mucosa of the colon is unable to obtain all its nourishment from the blood that reaches it; up to 80% of the energy is taken from the colonic lumen. Intraluminal microflora, acting on prebiotics such as undigested dietary fiber, mucoproteins contained in intestinal secretions, cells extruded from the colonic mucosa, and degraded bacteria or yeast, produces short-chain fatty acids; compounds such as hydrogen peroxide, lactic acid, amino acids, polyamines, vitamins (group B, folic acid and K), antioxidants, and growth factors. In vitro and in vivo studies in laboratory animals have shown that SCFAs have therapeutic potential: a) they regulate proliferation, differentiation, gene expression, and immune function at the colonic mucosa; b) they help superficial colonic lesions to heal; and c) they may reduce the risk of colon cancer, as they decrease the production and increase the degradation of procarcinogenic or mutagenic substances (33).
Probiotic fermentation produces intraluminal gases (H2, CO2 and CH4). If it is exaggerated or unaltered, it causes abdominal pain or discomfort and flatulence, which brings patients a great deal of discomfort. A number of commensal bacteria have methanogenic properties: Veillonella spp. (especially V alcalescens) and Clostridia spp. Some authors claim that IBS patients have chronic intestinal bacterial overgrowth, but this has only been found by Dr Pimentel's group in Los Angeles (34) and not by others (35), and it has also been correlated with the long-term use of PPIs (36), which is very common in this type of patient (3).
Intestinal microflora
Intestinal microflora is a complex ecosystem that is involved in the physiological functions of the host organism. It can be intraluminal or planktonic (80% of the dry mass of feces), or mucosa-associated microbiota (MAM). The functions of these are different, as shown in table IV. The human being accommodates approximately 100 billion intestinal bacteria (10 times more prokaryotic cells than eukaryotic cells in the body) from about 17 families, 50 genera, 400-500 different species and an undefined number of subspecies. Of all these bacteria, 99% are anaerobic. It has not been possible to culture most of them (32).
The distribution of bacteria in the digestive tract is different for different sections, the largest number being in the buccal cavity and the large intestine. The stomach contains fewer than 103 microorganisms per ml, whilst the duodenum and jejunum contain around 105/ml, the main ones being Clostridium perfringens and Bacteroides fragilis; the fundamental factor that controls duodenojejunal intestinal microflora is the maintenance of peristalsis. Beyond the terminal ileum there is an increase in colonization, which reaches 107-10/ml and is 1011-12/ml in the colon, with both planktonic bacteria and MAM, whose habitat is the viscous mucus at the mucosal surface. The main genera of bacteria are: Lactobacillus, Bifidobacteria, Bacteroides, Clostridia, Fusobacteria, Eubacteria, Peptococcus, Streptococcus, Escherichia and Veillonella; as a percentage, MAM has fewer anaerobes than planktonic intestinal microflora. The normal balance of intestinal microflora can be affected by a number of factors, and this in turn causes certain functional disorders (Table V). There is evidence that intestinal microflora plays a significant role in intestinal inflammation by altering the bowel; we also know that in animals that are germ-free since birth the development of experimental intestinal inflammation is prevented or minimized (37).
Probiotics
The concept of probiotic ("for life") is 100 years old now, since Elie Metchnikoff published "The prolongation of life: optimistic studies" in 1907. In 1998 F Guarner defined probiotics as living microorganisms that have benefits for the gastrointestinal tract and its immune function after being ingested. In 2003 the concept of "immunobiotic" was introduced, including those that modulate the immune response throughout the MALT system; this idea maintains that the intestinal mucosa and intestinal microflora constitute an anatomical-functional unit that regulates both the cell-mediated and humoral immune responses and the local production of cytokines. In 2008 the WGO published an excellent review on prebiotics and probiotics, which can be consulted online (38).
Probiotic bacteria are Lactobacilli spp., certain types of Streptococcus, and Bifidobacteria spp., but also other non-pathogenic bacilli such as E. coli-Nisle1917 and yeasts such as Saccharomyces boulardii. The best known and most widely used probiotics are Lactobacillus plantarum 299v, Lactobacillus rhamnosus LGG, Lactobacillus reuteri, Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium infantis, lactis or brevis. Probiotics can be administered not only as functional foods, but also in pharmaceutical forms similar to medicines, which were then called nutraceuticals.
Probiotics have various actions: a) physical barrier effect; b) competition for nutrients; c) metabolic interactions; d) bacteriocin production; e) reinforcement of the intestinal mucosal barrier; f) reduction of intestinal permeability and bacterial translocation; and particularly, g) regulation of the intestinal inflammatory response by modulating the secretion (local and systemic) of cytokines and the immune response (local and general). For a probiotic to be effective, five conditions must be fulfilled: 1) it must have a proven beneficial effect on the host; 2) it must not be toxic or pathogenic; 3) it must contain a sufficiently large number of viable microorganisms per unit; 4) it must be capable of surviving in the intestine, reproducing, maintaining itself, and having intraluminal metabolic activity; and 5) it must remain viable during storage and use. The mechanisms of action of probiotics are shown in table VI, and their general benefits are shown in table VII. The main action of the protective properties of probiotics is carried out by DCs, antigen-presenting cells for T lymphocytes that are found in mucosas, lymphoid tissues, lymph, lymph nodes, spleen and peripheral blood, although in a smaller quantity here because they make up less than 2% of mononuclear cells. DC phenotype and cytokine production is modulated by the intestinal microflora; furthermore, these cells are involved in the local immune response to B lymphocyte activation and IgA synthesis by plasmacytes.
The evidence is type I (grade A) for the use of probiotics in the treatment of lactose malabsorption and acute infectious diarrhea in children, the prevention of nosocomial diarrhea and antibiotic-associated diarrhea, as well as the prevention of pouchitis in colectomized ulcerative colitis patients. However, evidence is type II (grade B) in the prevention of traveller's diarrhea, radiation colitis, maintenance of clinical remission in ulcerative colitis, and treatment of abdominal pain, constipation and distension in IBS. In the latter case it is because not all probiotics have been shown to be equally effective, and there are still relatively few studies, some of which are not randomized, some with a single probiotic, others with multiple species, and even some combined with prebiotics. One of the probiotics with more extensive experience and better results in IBS is Lactobacillus plantarum (Lp). These general points were also made in the paper by Dr F Vargas "Probiotics in Gastroenterology", which was presented on 18-11-08 during the National Gastroenterology Week at the Congress of the Mexican Gastroenterology Association (39), which analyzed the relative risk (RR), attributable risk (AR), and the necessary number of subjects to be treated to achieve a significant benefit (NNT = 1/AR), which indicates that the intervention is cost-beneficial if it is below 10, in all the different actions of probiotics that have been published in the last 12 years, also specifying those that give the best results in clinical trials with methodological quality. All of this is extensively presented in table VIII.
Clinical Practice Guideline No. 5 (40): "Irritable Bowel Syndrome" in the Treatment section concluded that "...probiotics could improve the global symptoms of IBS, based on two positive RCTs, compared to three RCTs that did not show any effectiveness"; in recent years there have been numerous publications (41-45), which have refined our therapeutic approach. The bases for this were provided by studies showing that the intestinal microflora of these patients differs from that of healthy controls (46,47), at least in a subgroup of IBS patients (11). When ribosomal RNA-based microbiological technologies were used with cloning and sequencing of genes (27), these differences were even more significant. This would have a certain role in its etiopathogenesis, as the balance between the intestinal microflora, epithelium and gut-associated lymphoid tissue (GALT) plays a fundamental role in healthy intestinal homeostasis and in the pathophysiology of certain intestinal or systemic diseases (48). Host-intestinal microflora interactions lead to immune tolerance, sustain the function and integrity of the epithelial barrier and its vascularization, promote the development and maintenance of GALT, and are essential for the development of jejuno-colonic motility under physiological conditions. The role of probiotics in IBS and their possible mechanisms of action were analyzed by M Camilleri (49,50), who concluded that probiotics are a promising therapy for IBS. This is justified by the fact that treatment with probiotics alters certain mechanisms of IBS: a) reduction of intracolonic gas of bacterial origin due to an increase in Lactobacilli and Bifidobacteria, and a consequent decrease in the proportion of Clostridia and Veillonella; b) increase in the production of intracolonic SCFA and a consequent improvement in colonic propulsion; c) reduced malabsorption of bile acids in diarrhea-predominant IBS, because Lactobacilli and Bifidobacteria are capable of deconjugating and absorbing bile acids, thus reducing luminal load, which reduces colonic mucorrhea and hydrorrhea; and d) production of anti-inflammatory changes and normalization of intestinal immunity, preventing intestinal permeability and thus reducing bacterial translocation; it decreases serotonin-secreting ECL cells; and it regulates the action of mastocytes in the vicinity of nerve endings within the intestinal lamina propria.
Treatment of IBS with probiotics
Probiotics reinforce the intestinal mucosal barrier, inhibit the mucosal adhesion of pathogens and alter the intestinal inflammatory response, normalizing the motility of the digestive tract and its visceral sensitivity. They may also regulate intraluminal fermentation and stabilize the intestinal microflora. In the words of R Spiller (51): "Before 2008 it could be concluded that probiotics were very safe but not very effective in the treatment of IBS. After 2008, there is reasonable evidence of the symptomatic benefits for pain, bowel habit, and reduction of flatulence and distension in IBS patients, which is particularly noticeable in RCTs with a larger number of patients. In any case, it must be taken into account that IBS patients are heterogeneous and each probiotic has unique features". There are also two underlying questions: 1) should we use a specific probiotic or multiple species?; and 2) in the form of functional foods or as a nutraceutical?
Probiotic bacteria are defined at three levels: genus, species and strain; it is essential to understand that their properties depend on all three and cannot be assigned to other similar ones, even if they share the same genus and species. In other words, the therapeutic benefits of a strain of probiotics cannot be extended to other strains within the same species, and their efficacy has to have been proven individually. Therefore, we shall now examine evidence available to date.
Studies with one probiotic
Lactobacillus
This genus contains over 125 species, which are used industrially in the production of yoghurt, cider, wine, sauerkraut, pickles, cheese, chocolate, and other fermented foods. Lactobacillus plantarum (Lp) is found in fermented foods, pickles, stored cereals and fodder fermented in silos. It is gram+, microaerophilic, facultatively heterofermentative and produces hydrogen peroxide; it grows at 15º, but not at 45º, and produces both isomers of lactic acid from lactose. It has a great ability to colonize and survive at both ends of the human digestive tract, which explains its proportional presence in the saliva and feces (52). Lp299v was identified in Lund and was patented in the USA in 1995, together with L. rhamnosus 271 (DSM 6594), which was discovered by the same group (53). The mucosal binding and colonization ability and tolerance to pH and bile, tested on 46 strains of Lactobacilli, showed that Lp 299v resists pH 2.5 and the effect of bile for at least 4 hours, and is 4th in terms of mucosal binding ability (54). In 2001 Vanderhoof (55) highlighted the properties of different strains of Lactobacillus, especially Lp299v, mentioning their ability: a) to prevent inflammatory changes in methotrexate-induced enterocolitis in rats; b) to reduce IL10 production in the knockout mouse model; c) to regulate genes associated with mucin production in cell cultures; d) to effectively treat children with bacterial overgrowth due to short bowel syndrome; and e) to stimulate antibody production following the administration of an anti-rotavirus vaccine, a feature that is not shared with other strains of Lactobacillus.
In the treatment of IBS, most studies published with Lp299 obtained significant improvements compared with other treatments or placebo. Niedzielin et al. (56) showed a significant improvement in abdominal pain in 100 IBS patients, compared with placebo or spasmolytics. The same authors (57) analyzed the response in 40 IBS patients (80% women) who were symptomatic at that moment and were being treated with different therapies, and who had been referred from primary care; they achieved twice the abdominal pain relief versus placebo, normalization of bowel habit in 60% of constipation-predominant IBS (18% with placebo), and a resolution of flatulence in 55% (33% with placebo). Nobaek et al. (29) showed that, in 60 patients with IBS, abdominal distension and flatulence, there was a significant reduction in overall discomfort in 44% of patients on Lp299v (18% with placebo); this improvement continued for up to one year after stopping Lp299v. E. Hauschildt showed in laboratory animals that this probiotic reduces bacterial translocation and improves the physiology of the intestinal and hepatic mucosa (58); it also increases carboxylic acid levels in feces and reduces abdominal distension. As an example of their anti-inflammatory properties the author pointed out their ability to reduce blood fibrinogen. However, Sen et al. (59) did not achieve a significant response to abdominal pain with 12 IBS patients (the RCT with the lowest number of cases of all) after administering Lp299v versus a placebo, although there was a significant reduction in the production of colonic gas (more than 50%) after taking the probiotic.
Other Lactobacilli have been studied: O'Sullivan and O'Morain (60) used a double-blind placebo-controlled analysis in 24 patients with Rome II-defined IBS (80% women; aged 18-75) to assess the effect of Lactobacillus casei GG in enteric-coated tablets for eight weeks on abdominal pain and distension, and defecation urgency, without finding significant differences; only 19 of the 24 patients completed the study, which is too small a number to draw conclusions. In 40 patients aged 18-70 who met Rome III criteria, the administration of Lactobacillus acidophilus versus placebo for 4 weeks improved abdominal pain/discomfort by 24%, also increasing their perception of a better quality of life (61).
Bifidobacteria
In 274 primary care patients with constipation-predominant IBS, the administration of yoghurt + Bifidobacterium animalis compared with heat-treated yoghurt as placebo for six weeks brought about an improvement in abdominal pain and bloating, constipation and quality of life after the 3rd week (62). With this same strain it has been published that the administration of yoghurt containing Bifidobacterium lactis DN-173010 for 4 weeks in 34 women with constipation-predominant IBS reduced abdominal pain, abdominal distension (measured using a measuring tape), volume of gas produced (measured by ambulatory plethysmography), oro-cecal transit time (H2 breath test) and colonic transit time (radiopaque markers) (63). Although both constipation-predominant IBS and abdominal distension are much more common in women than in men, the latter study (randomized, double-blind, controlled) was carried out in female patients only, and it is yet to be verified whether these results would have occurred in male patients.
Previously, a multi-center study (in 20 centers in the United Kingdom) in 362 patients with Rome II-defined IBS from primary care (90% women), aged 19-69, who received Bifidobacterium infantis 35624 in capsule form for 4 weeks at three different doses (106, 108 and 1010 CFUs), showed that there was an improvement in abdominal pain/discomfort that was 20% greater than with placebo, but only at doses of 108 CFUs/day (64).
Studies with several species of probiotics
In 2004 Saggioro showed that in 70 IBS patients (56% women) both the Lp + L. acidophilus and Lp + Bifidobacterium brevis combinations, compared to a starch placebo, improved symptoms by 45-56% after both 4 and 8 weeks (65). Supplements with multiple species (2 Lactobacillus + Propionobacteria + Bifidobacteria animalis) stabilized the intestinal microflora in 86 IBS patients, improved their quality of life, relieved abdominal pain, and stopped constipation with no noticeable adverse effects (66).
There are two studies that assessed VSL#3 in the treatment of IBS. In one study, 25 patients with diarrhea-predominant IBS were included and randomized to receive 2 sachets of probiotics or placebo for 8 weeks (67); in those who received the active product there was no improvement in abdominal pain, colonic transit time, or quality of life, but a reduction was noted for abdominal distension and flatulence. The other study included 45 IBS patients whose main complaints were abdominal bloating/distension and who received the probiotic mixture or placebo for 4-8 weeks (68); results overlapped: improvement in abdominal distension and flatulence, but not in other parameters such as abdominal pain, quality of life, or bowel habit. In a paper presented at the AGA 2007 meeting, Simren et al. (69) used the combination of L. paracasei, L. acidophilus and Bifidobacterium lactis as a probiotic in yoghurt in 14 IBS patients, compared to 17 who received placebo, and found similar symptomatic relief in both, concluding no benefits with probiotics.
Meta-analysis
A thorough meta-analysis of 20 RCTs (1,404 subjects) shows that the use of probiotics was associated with overall improvement in IBS (RR = 0.77) and a reduction in abdominal pain episodes (RR = 0.78) (70). Of those 20 studies, the ones with the highest quality are three with Lp299v (29,56,57), three with Lactobacillus rhamnosus, with 24, 58 and 37 subjects each, and 6 with multiple species of probiotics. Similar conclusions were reached in a meta-analysis that identified 19 quality RTCs published between 1966 and 2008 (71), including the three with Lp299v mentioned above. Both claim that probiotics are beneficial in the treatment of IBS, but the extent of the benefit and the most effective species (or combinations) are still somewhat uncertain.
Safety of probiotics
Taking probiotics is becoming an increasingly important part in the diet of industrialized countries, as their general and gastrointestinal beneficial effects are being gradually proven. It has become necessary to harmonize marketing criteria, regulate health claims of functional foods, evaluate the efficacy of probiotics, correctly define what is and what is not a probiotic, what the effective doses are, and whether they are completely safe. The safe use of probiotics is an absolutely crucial matter. Different studies have shown that the use of probiotics in healthy subjects (72,73), and even in immunocompromised patients (74,75), involves a very low risk of bacterial complications, although over 80 cases of bacteremia have been reported in Finland, associated with severe prior comorbidities or surgery (76), and always with Lactobacillus. Lactobacilli, Bifidobacteria and other commensal microorganisms are considered GRAS ("Generally Regarded As Safe"), although certain doubts have been raised regarding their use at massive doses in immunodepressed patients or in those who undergo intestinal resection due to benign or malignant disease. Other microorganisms such as enterococcus may be opportunistic pathogens depending on host conditions, and should not be administered to immunocompromised patients.
References
1. Longstreth GF, Thomson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006; 130: 1480-91. [ Links ]
2. Lehrer JK, Lichtenstein GR. Irritable bowel syndrome. On line: eMedicine Specialties / Gastroenterology / Colon. Updated: Sep. 9, 2008. [ Links ]
3. Faresjö A, Grodzinsky E, Johansson S, Wallander MA, Faresjo T, Timpka T. Self-reported use of pharmaceuticals among patients with irritable bowel syndrome in primary care. J Manag Care Pharm 2008; 14: 870-7. [ Links ]
4. Gilkin RJ. The spectrum of irritable bowel syndrome: a clinical review. Clin Therap 2005; 27: 1696-709. [ Links ]
5. Hammer J, Eslick GD, Howell SC, Altiparmak E, Talley NJ. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut 2004; 53: 666-72. [ Links ]
6. Agrawal A, Whorwell PJ. Abdominal bloating and distension in functional gastrointestinal disorders - epidemiology and exploration of possible mechanisms. Aliment Pharmacol Ther 2008; 27: 2-10. [ Links ]
7. Hamaguchi T, Fukudo S, Kanazawa M, Tomiie T, Shimizu K, Oyama M, et al. Changes in salivary physiological stress markers induced by muscle stretching in patients with irritable bowel syndrome. Biopsychosoc Med 2008; 2: 20. [ Links ]
8. Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology 2001; 121: 799-804. [ Links ]
9. Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, et al. Serotonin transporter polymorphism pharmacogenetics in diarrea-predominant irritable bowel syndrome. Gastroenterology 2002; 123: 425-32. [ Links ]
10. Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut 2001; 48: 14-9. [ Links ]
11. Verdu E, Collins SM. Microbial-gut interactions in health and disease. Irritable bowel syndrome. Best Pract Res Clin Gastroenterol 2004; 18: 315-21. [ Links ]
12. Talley NJ. Irritable bowel syndrome. Intern Med J 2006; 36: 724-8. [ Links ]
13. Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007; 132: 913-20. [ Links ]
14. Zhang H, Yan Y, Shi R, Lin Z, Wang M, Lin L. Correlation of gut hormones with irritable bowel syndrome. Digestion 2008; 78: 72-6. [ Links ]
15. Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002; 122: 1778-83. [ Links ]
16. Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 2005; 115: 398-409. [ Links ]
17. Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitryptiline reduces rectal pain related activation of the anterior cingulated cortex in patients with irritable bowel syndrome. Gut 2005; 54: 601-7. [ Links ]
18. McNally MA, Locke GR, Zinsmeister AR, Schleck CD, Peterson J, Talley NJ. Biliary events and an increased risk of new onset irritable bowel syndrome: a population-based cohort study. Aliment Pharmacol Ther 2008; 28: 334-43. [ Links ]
19. Lim B, Manheimer E, Lao L, Ziea E, Wisniewski J, Liu J, Berman B. Acupuncture for treatment of irritable bowel síndrome. Cochrane Database Syst Rev 2006; 4: CD005111. [ Links ]
20. Harris LA, Hansel S, DiBaise J, Crowell MD. Irritable bowel syndrome and chronic constipation: emerging drugs, devices, and surgical treatments. Curr Gastroenterol Rep 2006; 8: 282-90. [ Links ]
21. Hussain Z, Quigley EMM. Systematic review: complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther 2006; 23: 465-71. [ Links ]
22. Jarcho JM, Chang L, Berman M, Suyenobu B, Naliboff BD, Lieberman MD, et al. Neural and psychological predictors of treatment response in irritable bowel syndrome patients with a 5-HT3 receptor antagonist: a pilot study. Aliment Pharmacol Ther 2008; 28: 344-52. [ Links ]
23. Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ 2008; 337: 1388-92. [ Links ]
24. Jones R. Treatment of irritable bowel syndrome in primary care. Ispaghula, antispasmodics, and peppermint oil should be considered. BMJ 2008; 337: 1361-2. [ Links ]
25. Fayyaz M, Lackner JM. Serotonin receptor modulators in the treatment of irritable bowel syndrome. Ther Clin Risk Man 2008; 4: 41-8. [ Links ]
26. Quigley EAA. Bacterial flora in irritable bowel syndrome: role in pathophysiology, implications for management. J Dig Dis 2007; 8: 2-7. [ Links ]
27. Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007; 133: 24-33. [ Links ]
28. King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet 1998; 352: 1187-9. [ Links ]
29. Nobaek S, Johansson ML, Molin G, Ahrne S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol 2000; 95: 1231-8. [ Links ]
30. Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil 2008; 20(Supl. 1): 121-9. [ Links ]
31. Salminen S, Isolauri E, Salminen E. Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful strains and future challenges. Antonie Van Leeuwenhoek 1996; 70: 347-58. [ Links ]
32. Bixquert M. Anatomía funcional del intestino delgado y del colon. En: Díaz-Rubio M, Rey E, editores. Trastornos motores del aparato digestivo 2ª ed. Madrid: Editorial Panamericana; 2007. p. 183-95. [ Links ]
33. Bharucha AE. Lower gastrointestinal functions. Neurogastroenterol Motil 2008; 20(Supl. 1): 103-13. [ Links ]
34. Lee HR, Pimentel M. Bacteria and irritable bowel syndrome: the evidence for small intestinal bacterial overgrowth. Curr Gastroenterol Rep 2006; 8: 305-11. [ Links ]
35. Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simren M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007; 56: 802-8. [ Links ]
36. Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102: 2047-56. [ Links ]
37. Strauch UG, Obermeier F, Grunwald N, Gürster S, Dunger N, Schultz M, et al. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut 2005; 54: 1546-52. [ Links ]
38. Guarner F, Khan AG, Garisch J, Eliakim R, et al. Guías prácticas de la OMGE. Probióticos y prebióticos. Mayo 2008. Disponible en: http://www.worldgastroenterology.org/assets/downloads/es/pdf/guidelines/19_probioticos_prebioticos_es.pdf. [ Links ]
39. Vargas F. Desayuno de trabajo: Probióticos en Gastroenterología. Congreso Mexicano de Gastroenterología, Veracruz, México, 18 de noviembre 2008. Comunicación personal. [ Links ]
40. Grupo de trabajo. Manejo del paciente con síndrome del intestino irritable. Guía de práctica clínica nº 5. Barcelona: AEG, SemFYC y Centro Cochrane Iberoamericano; 2005. [ Links ]
41. Sullivan A, Nord CE. Probiotics and gastrointestinal diseases. J Intern Med 2005; 257: 78-92. [ Links ]
42. Young P, Cash BD. Probiotic use in irritable bowel syndrome. Curr Gastroenterol Rep 2006; 8: 321-6. [ Links ]
43. NASPGHAN Nutrition Report Committee, Michail S, Sylvester F, Fuchs G, Issenman R. Clinical practice guideline. Clinical efficacy of probiotics: review of the evidence with focus on children. J Pediatr Gastroenterol Nutr 2006; 43: 550-7. [ Links ]
44. Borowiec AM, Fedorak RN. The role of probiotics in management of irritable bowel syndrome. Curr Gastroenterol Rep 2007; 9: 393-400. [ Links ]
45. Quigley EAA. Probiotics in the management of colonic disorders. Curr Gastroenterol Rep 2007; 9: 434-40. [ Links ]
46. Quigley EAA. Bacterial flora in irritable bowel syndrome: role in pathophysiology, implications for management. J Dig Dis 2007; 8: 2-7. [ Links ]
47. Quigley EAA, Flourie B. Probiotics and irritable bowel syndrome: a rationale for their use and assessment of the evidence to date. Neurogastroenterol Motil 2007; 19: 166-72. [ Links ]
48. Quigley EAA. Probiotics in functional gastrointestinal disorders: what are the facts. Curr Op Pharmacol 2008; 8: 704-8. [ Links ]
49. Camilleri M. Is there a role for probiotics in irritable bowel syndrome? Dig Liv Dis 2006; 38(Supl. 2): S266-S269. [ Links ]
50. Camilleri M. Probiotics and irritable bowel syndrome: rationale, putative mechanisms, and evidence of clinical efficacy. J Clin Gastroenterol 2006; 40: 264-9. [ Links ]
51. Spiller R. Review article: probiotics and prebiotics in inrritable bowel syndrome. Aliment Pharmacol Ther 2008; 28: 385-6. [ Links ]
52. Maukonen J, Mättö J, Suiko ML, Saarela M. Intra-individual diversity and similarity of salivary and faecal microbiota. J Med Microbiol 2008; 57: 1560-8. [ Links ]
53. Johansson ML, Molin G, Jeppsson B, Nobaek S, et al. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl Environm Microb 1993; 59: 15-20. [ Links ]
54. Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, Møller PL, Michaelsen KF, Paerregaard A, et al. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol 1999; 65(11): 4949-56. [ Links ]
55. Vanderhoof JA. Probiotics: future directions. Am J Clin Nutr 2001; 73(Supl.): 1152S-1155S. [ Links ]
56. Niedzelin K, Kordecki H, Kosik R. New possibility in the treatment of irritable bowel syndrome: probiotics as a modification of the microflora of the colon. Gastroenterology 1998; 114: A402. [ Links ]
57. Niedzelin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 2001; 13: 1143-7. [ Links ]
58. Hauschildt E. Lactobacillus plantarum 299v reduces irritable bowel bloating. Am J Clin Nutr 2001; 73: 380S-385S. [ Links ]
59. Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci 2002; 47: 2615-20. [ Links ]
60. O'Sullivan MA, O'Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomized double-blind placebo-controlled crossover study. Digest Liver Dis 2000; 32: 294-301. [ Links ]
61. Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, et al. Therapeutic effect of Lactobacillus acidophilus SDC 2012,2013 in patients with irritable bowel syndrome. Dig Dis Sci 2008; 53: 2714-8. [ Links ]
62. Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther 2007; 26: 475-86. [ Links ]
63. Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2008; 29: 104-14. [ Links ]
64. Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006; 101: 1581-90. [ Links ]
65. Saggioro A. Probiotics in the treatment of irritable bowel syndrome. J Clin Gastroenterol 2004; 38: S104-S106. [ Links ]
66. Kajander K, Myllyluoma E, Rajili?-Stojanovi? M, Kyrönpalo S, Rasmussen M, Järvenpää S, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther 2008; 27: 48-57. [ Links ]
67. Kim HJ, Camilleri M, McKinzie S, Lempke MB, et al. A randomized controlled trial of a probiotic,, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2003; 17: 895-904. [ Links ]
68. Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, et al. A randomized controlled trial of a probiotic combination VSL#3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil 2005; 17: 687-96. [ Links ]
69. Simren M, Lindh A, Sammelsson L, Olsson J, Posserud I, Strid H, et al. Effect of a yoghurt containing three probiotic bacteria in patients with irritable bowel syndrome (IBS) - A randomized, double-blind, controlled trial. Gastroenterology 2007; 132(Supl. 2): A210. [ Links ]
70. Mcfarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol 2008; 14: 2650-61. [ Links ]
71. Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein A, Brandt L, et al. The efficacy of probiotics in the therapy of irritable bowel syndrome: a systematic review. Gut 2008; ePub ahead of print: doi:10.1136/gut.2008.167270. [ Links ]
72. Wolf BW, Garleb KA, Ataya DG, Casas IA. Safety and tolerance of Lactobacillus reuterii in healthy adult male subjects. Microbial ecology in health and disease 1995; 8: 41-50. [ Links ]
73. Salminen MK, Tynkkynen S, Rautelin H, Saxelin M, Vaara M, Ruutu P, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis 2002; 35(10): 1155-60. [ Links ]
74. Salminen MK, Tynkkynen S, Rautelin H, Poussa T, et al. The efficacy and safety of probiotic Lactobacillus rhamnosus GG on prolonged, noninfectious diarrhea in HIV patients on retroviral therapy: a randomized, placebo-controlled, crossover study. HIV Clin Trials 2004; 5: 183-91. [ Links ]
75. Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L rhamnosus GG. Clin Infect Dis 2004; 38: 62-9. [ Links ]
76. Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, et al. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis 2006; 42: e35-44. [ Links ]
![]() Correspondence:
Correspondence:
Miguel Bixquert Jiménez.
Avda. Gola de Puchol, 6, torre A, pta. 18.
46012 El Saler. Valencia, Spain.
e-mail:Miguel.Bixquert@uv.es
Received: 23-02-09.
Accepted: 27-02-09.