My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.101 n.8 Madrid Aug. 2009
LETTERS TO THE EDITOR
Atypical pyoderma gangrenosum in inflammatory bowel disease. A severe diagnostic challenge
Pioderma gangrenoso atípico en enfermedad inflamatoria del intestino. Un problema diagnóstico
Key words: Pyoderma gangrenosum. Inflammatory bowel disease.
Dear Editor,
Pyoderma gangrenosum (PG) is a rare inflammatory and ulcerative disease of the skin of unknown etiology. Diagnosis of PG is based on clinical and histological data and requires the exclusion of other ulcerative diseases of the skin. There is an atypical form of PG that appears generally associated with hematological malignancies and rarely has been described in patients with inflammatory bowel disease (IBD). We describe the case of a patient diagnosed with IBD in whom symptomatology was mainly extraintestinal as atypical PG.
Case report
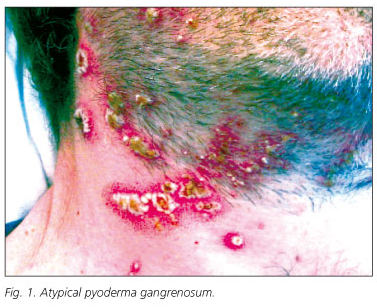
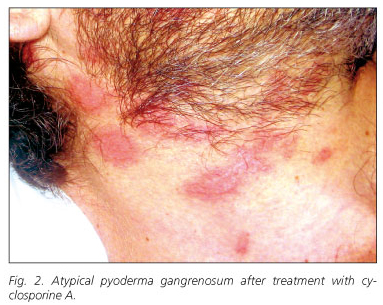
A 39-year-old man was referred to our hospital complaining of diarrhea consisting in passage of six to eight liquid stools containing mucus and small amounts of blood each day. One year before, he had been diagnosed of IBD, likely ulcerative colitis, and was treated with corticosteroids and topical 5-aminosalicilates. At the time of hospitalization, in addition to diarrhea, he showed fever (38 ºC), anemia, leukocytosis and elevated reactive C protein serum levels (20.4 ng/mL). Physical examination revealed the presence of a perianal fistula. The colonoscopy demonstrated hyperemia, edema, friability, and granularity, as well as pinpoint ulceration of the colonic mucosa. These changes were diffuse and extended from the rectum throughout the left colon. Histologicaly, it displayed changes consistent with IBD. The patient was treated with high doses of intravenous corticosteroids (1 mg/kg/day of methylprednisolone) and broad-spectrum antibiotics. Intestinal symptomatology improved but acute phase reactants persisted elevated. During tapering of corticosteroid doses, the patient developed a skin rash consisting of pustules distributed through the face and neck. These lesions were initially interpreted as a steroidal acne eruption. In the following days, new lesions of similar characteristics appeared in a diffuse pattern, but mostly in face, neck, upper extremities and presternal region. These lesions were generally round, smaller than 1 cm, with a purulent content and surrounded by an erythematous halo. These lesions progressively gathered until forming ulcers of up to 3 cm with brown exudates (Fig. 1). Simultaneously, joint swelling, pain and several erytemathous nodules in the pretibial area appeared. Biopsies of the ulcers showed a neutrophilic infiltrate limited to the epidermis, forming a sterile subcorneus pustulosis. Bacterial and fungal cultures were negative. The clinical and histological picture suggested an atypical PG. Pathergy phenomenon, consisting in the reappearance of lesions at the point of scratching and biopsy, was also observed (2,3). Since these ulcers did not heal using maximal doses of corticosteroids, immunosuppressive therapy was considered. Serological markers of hepatotropic viruses were negative. A positive Mantoux test contraindicated the immediate administration of anti-TNFα therefore, latent tuberculosis treatment with isoniacide was started. Instead of anti-TNFα therapy, intravenous treatment with cyclosporine A was initiated and corticosteroids were tapered. After a few days of treatment, the lesions dried up and recovered their normal epithelium. They acquired a pale, pinkish color (Fig. 2). Oral cyclosporine A was used as maintenance treatment. Even though the patient had been previously diagnosed with ulcerative colitis, demonstration of small intestinal involvement and the presence of perianal fistulas suggested that the patient was really affected by Crohn's disease.
Discussion
PG was initially described by Brunsting, Goekerman and O'Leary in 1930 (4). The etiology is unknown but in 50% of the cases, it is associated with systemic disease, mainly with IBD, polyarthritis, ankylosing spondylitis, hematological malignancies (tricholeukemia, acute myeloblastic leukemia), monoclonal gammapathies, autoimmune hepatitis, systemic lupus erythematosus, Sjögren's syndrome, Beçet's syndrome, Sweet's syndrome, or other autoimmune disorders. The prevalence of classic PG in ulcerative colitis ranges from 0.5 to 5% of the cases. It has a universal distribution and is more frequent in females from 20 to 50 years of age. Even though some studies suggest that PG is mainly associated with UC (8), other studies did not confirm these data. PG can appear before, during or after the systemic disease. Its etiopathogenesis is still unknown but a defect in neutrophil quimiotaxis seems to be involved. Pathergy is present in 30% of the cases. Activity of cutaneus lesions associates with the intestinal involvement, but their course is independent. When PG associates with a rheumatologic disease, its course seems more severe and active, but the reason for these differences is unknown.
Other forms of PG have been described (5). The typical or ulcerative form usually starts with very small papules or pustules with an erythematous base that rapidly extend. Ulceration and necrosis of the border of the ulcers occur. The majority of the typical forms are localized in the inferior extremities, mainly in the pretibial region. Normally, it heals leaving an atrophic and pinkish scar. PG lesions consist in a massive neutrophilic infiltration of the dermis, with hemorrhage and necrosis of the epidermis. Neutrophilic infiltrates are seen around and even inside the vessels, but no other signs of vasculitis can be found. The infiltrates can be mixed, lymphocytic and neutrophilic, mostly in the earlier phases (6). The atypical form of PG usually is found in the upper extremities, in the dorsum of the hands or in the face and neck. It is frequently associated with hemorrhage, bulla, and systemic features like fever, myalgias and arthralgias. Generally it is seen in the context of hematological malignancies (7). Other characteristic feature of this form of PG is the superficiality of the neutrophilic infiltrate, which is mainly confined to the epidermis. Some lesions are vesiculo-bullous, but it is not a mandatory feature of this form of PG. In the present case, the lesions predominated in the upper extremities, face and chest, and the infiltrate of polimorphonuclear cells had a subcorneal location. The pustular form of PG appears as little pustulas that converge and, eventually, can evolve into the typical form. It is frequently associated with IBD and Beçet's syndrome. The last form of PG, the superficial granulomatous form, has a minimal neutrophilic infiltration and has a less severe course. It has been described in patients with Wegener's disease, and usually follows traumatisms and thoracic surgery (8-10). Pyostomatosis vegetans is a variety of PG localized in the oral mucosa and is normally associated with IBD.
Differential diagnosis of PG includes multiple diseases such as arterial diseases, vasculitis, carcinomas, infections, insect bites, Wegener's disease, tuberculosis, syphilis, and particularly Sweet's syndrome. The latter syndrome is an acute and febrile condition consisting in a severe neutophilic dermatosis that occasionally appears in patients with IBD. This syndrome is also found in patients with connective disorders or hematological malignancies like monoclonal gammapathy, myeloid metaplasia and polycythemia vera. It normally forms erythematous, polycyclic, edematous, and painful plaques situated in the head, neck, and arms that are accompanied by fever and leukocytosis. These lesions also had an intense neutrophilic infiltration of the dermis without features of vasculitis. In most occasions it resolves with corticosteroids, thalidomide or dapsone (11). In cases of PG, it is necessary to rule out bacterial, mycobacterial, viruses, or fungi infection; therefore, culture of the tissue is mandatory. In all forms of PG, pathergy can be found (12).
Pharmacological treatment of election, apart from local therapy (with potent topical corticosteroids (13), tacrolimus, pimecrolimus and topical antibiotics), is intravenous pulses of high doses of methylprednisolone until a response is obtained. In the most severe and corticorefractary cases, cyclosporine A, tacrolimus, azathioprine, or micophenolate mofetil, ciclophosphamide, clorambucil, intravenous gammaglobulin, thalidomide, etanercept, Infliximab, adalimumab, clofazimine, dapsone, metronidazole and nicotine have been used (14-18). Some studies have shown that treatment of resistant cases can be done with other immunosuppressants. Thus, 69% of patients treated with Infliximab respond to this therapy whether or not IBD is associated with PG. A local treatment with extreme hygiene is necessary to avoid bacterial over infection. In refractory cases, hyperbaric oxygen can be used. It is frequent that PG lesions recur during follow-up of patients with IBD. Surgical debridement is not recommended because of the high risk of pathergy.
P. Solís Muñoz, A. Franco Ugidos, P. Martínez Montiel, T. Muñoz Yagüe, C. Garrido1 and J. A. Solís Herruzo
Departments of Gastroenterology and Hepatology, and 1Dermatology. Universitary Hospital "12 de Octubre". Madrid, Spain
References
1. Lewis SJ, Poh-Fitzpatrick MB, Walther RR. Atypical pyoderma gangrenosum with leucemia. JAMA 1978; 239: 935-8. [ Links ]
2. Patel P, Topilow A. Atypical pyoderma gangrenosum in a splenectomy incision. New Eng J Med 2002; 347: 1419. [ Links ]
3. Gulyas K and Kimble FW. Atypical pyoderma gangrenosum after breast reduction. Aesth Plast Surg 2003; 27: 328-31. [ Links ]
4. Perry HO, Brunsting LA. Pyoderma gangrenosum: a clinical study of 19 cases. AMA Arch Dermatol 1957; 75: 380-6. [ Links ]
5. Armstrong PM, Ilyas I, Pandey R, Berendt AR, Conlon CP, Simpson AH. Pyoderma gangrenosum. A diagnosis not to be missed. J Bone Joint Surg Br 1999; 81: 893-4. [ Links ]
6. Powell FC, Schroeter AL, Su WP, Perry HO. Pyoderma gangrenosum: a review of 86 patients. Q J Med 1985; 55: 173-86. [ Links ]
7. Hay CR, Messenger AG, Cotton DW, Bleehen SS, Winfield DA. Atypical bullous pyoderma gangrenosum associated with myeloid malignancies. J Clin Pathol 1987; 40: 387-92. [ Links ]
8. Prystowsky JH, Khan SN, Lazarus GS. Present status of pyoderma gangrenosum. Review of 21 cases. Arch Dermatol 1989; 125: 57-64. [ Links ]
9. Von den Driesch P. Pyoderma gangrenosum: a review of 44 cases with follow-up. Br J Dermatol 1997; 137: 1000-5. [ Links ]
10. Harris AJ, Regan P, Burge S. Early diagnosis of pyoderma gangrenosum is important to prevent disfigurement. BMJ 1998; 316: 52-3. [ Links ]
11. Bennett ML, Jackson M, Jorrizo JL, Fleischer AB, White WL, Callen JP. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine 2000; 79: 37-46. [ Links ]
12. Zivanovic D, Tanasilovic S, Skiljevic D, Tomovic M, Bogdanovic A, et al. Atypical pyoderma gangrenosum in a patient with osteomyelofibrosis. Vojnosanit Pregl 2007; 64: 787-9. [ Links ]
13. Sams WM Jr. Inflammatory ulcers. In: Sams WM Jr, Lynch PJ, editors. Principles and practice of dermatology. 2nd ed. New York: Churchill Livingstone; 1996. p. 917-9. [ Links ]
14. Newell LM, Malkinson FD. Pyoderma gangrenosum. Response to cyclophosphamide therapy. Arch Dermatol 1983; 119: 495-7. [ Links ]
15. Penmetcha M, Navaratnam AE. Pyoderma gangrenosum. Response to cyclosporin A. Int J Dermatol 1988; 27: 253. [ Links ]
16. Curley RK, Macfarlane AW, Vickers CF. Pyoderma gangrenosum treated with cyclosporin A. Br J Dermatol 1985; 113: 601-4. [ Links ]
17. Teitel AD. Treatment of pyoderma gangrenosum with methotrexate. Cutis 1996; 57: 326-8. [ Links ]
18. López San Román A, Bermejo F, Aldanondo I, Carrera E, Boixeda D, Muñoz Zato E. Pyoderma gangrenosum associated with ulcerative colitis: response to infliximab. Rev Esp Enferm Dig 2004; 96: 420-4. [ Links ]