Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.103 no.10 Madrid Out. 2011
https://dx.doi.org/10.4321/S1130-01082011001000015
LETTERS TO THE EDITOR
Romiplostim in chronic liver disease with severe thrombocytopenia undergoing an elective invasive procedure
Utilidad del romiplostim en la trombocitopenia asociada a cirrosis antes de un procedimiento invasivo
Key words: Romiplostim. Thrombocytopenia. Liver cirrhosis. Jehovah 's witness. Hepatocellular carcinoma.
Palabras clave: Romiplostim. Trombocitopenia. Testigo de Jehová. Carcinoma hepatocelular.
Dear Editor,
Thrombocytopenia is a common complication seen in patients with chronic liver disease, precluding or interfering invasive diagnostic and therapeutic procedures. Etiology is multifactorial, including hypersplenism, bone marrow suppression by HCV and a possible reduction in the level or activity of the hematopoietic growth factor thrombopoietin (TPO) (1). In addition to thrombocytopenia, functional platelet defects has been suggested. Romiplostim (Nplate®, Amgen Inc, Thousands Oaks, Calif) is a new thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in patients with chronic immune thrombocytopenic purpura who have had an insufficient response to corticosteroids, immunoglobulin or splenectomy (2). It has been not tested in chronic liver disease until now. Another thrombopoietin receptor agonist, eltrombopag (3), has been tested in chronic liver disease undergoing an elective invasive procedure. Although results were satisfactory in terms of platetelet count and avoidance of platelet transfusions, the trial was early stopped due to the observed incidence of portal vein thrombosis in the study group (4). We report a case of a Jehovah's Witness that could successfully managed with romiplostim use.
Case report
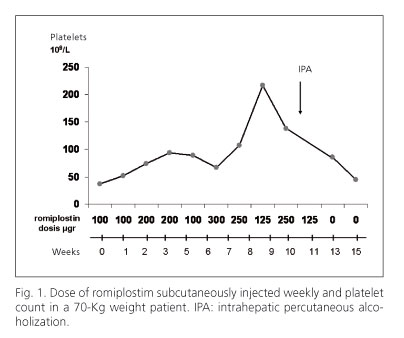
A 62-years-old male with liver cirrhosis secondary to HCV was diagnosed of a 30 mm hepatocellular carcinoma in segment IVB in contact with left portal vein by two radiological tests. The patient was Child A with 30 x 109 platelets/L. A trans-arterial chemo-embolization procedure was planned to treat his tumor. After two procedures a complete necrosis was not achieved and viable tumor was showed by CT. After informed consent, romiplostim therapy was begun as compasive use, treating to improve the platelet count to perform a radiofrequency ablation. The romiplostim dose was weekly varied depending on the platelet count. We planned the procedure after three doses of romiplostim but it was delayed because the increase on platelet count was insufficient to safely perform the procedure. The dose of romiplostim subcutaneous weekly use and the platelet count evolution are shown in figure 1. After 10 weeks of therapy a percutaneous ethanol injection (PEI) with a single-shot technique was performed. Immediately after the procedure an ultrasound with contrast showed no vascularization of the nodule and a partial thrombosis of the left portal vein in contact with the tumor. Anticoagulation with tinzaparine was begun. At four weeks after PEI, a new CT showed permeability of portal veins and a complete necrosis of the nodule without hypervascularization.
There are some concerns about romiplostim use: worsening thrombocytopenia after drug discontinuation and thrombotic events related to an elevated number of platelets.
In our patient, platelet count decreased slowly to previous figures after discontinuation of therapy. A partial thrombosis of the portal vein in contact with the treated area appeared during the procedure but disappeared after 4 weeks of anticoagulant therapy with a low-molecular weight heparin. We think it was probably related to an unnoticed injection of ethanol in the portal vein and not related to romiprostim use. Useful and safe thrombopoietin receptor agonists are needed in different clinical situations for chronic liver disease patient management; in our case we could successfully treat this patient, Jehovah's witness, who rejected platelet transfusion. Well designed clinical trials with romiplostim are required to study its use in this setting.
José Castellote, Anna Girbau, Claudia Arajol and Xavier Xiol
Unit of Hepatology and Liver Transplant. IDIBELL. Hospital Universitari de Bellvitge.
L'Hospitalet de Llobregat, Barcelona.. Spain
References
1. Afdhal N, McHutchison J, Brown R, Jacobson I, Mans M, Poordard F, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol 2008;48:1000-7. [ Links ]
2. Frampton JE, Lyseng-Williamson A. Romiplostim. Drugs 2009;69:307-17. [ Links ]
3. Zekry A, Freiman J. Eltrombopag: is this "24 karat gold platelet" treatment for thrombocytopenia in cirrhosis associated with hepatitis C? Hepatology 2008;47:1418-21. [ Links ]
4. Afdhal N, Giannini E, Tayyab GN, Mohsin A, Lee JW, Andriulli A, et al. Eltrombopag in chronic liver disease patients with thrombocytopenia undergoing an elective invasive procedure: results from ELEVATE, a randomised clinical trial (abstract). J Hepatol 2010;52:S460. [ Links ]