Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.104 no.6 Madrid jun. 2012
https://dx.doi.org/10.4321/S1130-01082012000600006
The role of endoscopic ultrasound (EUS) in relation to other imaging modalities in the differential diagnosis between mass forming chronic pancreatitis, autoimmune pancreatitis and ductal pancreatic adenocarcinoma
Papel de la endoscopia en relación con otras modalidades de imagen en el diagnóstico diferencial entre pancreatitis crónica en forma de masa, pancreatitis autoinmune y adenocarcinoma pancreático
Julio Iglesias-García1, Björn Lindkvist2, José Lariño-Noia1 and J. Enrique Domínguez-Muñoz1
1Department of Gastroenterology and Foundation for Research in Digestive Diseases (FIENAD). Hospital Universitario de Santiago de Compostela. A Coruña, Spain
2Institute of Medicine. Sahlgrenska Academy. University of Gothenburg. Gothenburg, Sweden
Disclosures: Dr. Julio Iglesias-García is international advisor of Cook-Medical. Dr. J. Enrique Domínguez-Muñoz is international advisor of Pentax Medical Company.
ABSTRACT
Differential diagnosis of solid pancreatic lesions remains as an important clinical challenge, mainly for the differentiation between mass forming chronic pancreatitis, autoimmune pancreatitis and pancreatic adenocarcinoma. Endoscopic ultrasound (EUS), computed tomography (CT) and magnetic resonance imaging (MRI) can all provide valuable and complementary information in this setting. Among them, EUS has the unique ability to obtain specimens for histopathological diagnosis and can therefore play a crucial role in the evaluation patients with inconclusive findings on initial examinations. Nowadays, new developed techniques associated to EUS, like elastography and contrast enhancement, have shown promising results for the differential diagnosis of these pancreatic lesions.
Key words: Endoscopic ultrasound. CT scan. MRI. Pancreatic tumors.
RESUMEN
El diagnóstico diferencial de las lesiones sólidas pancreáticas permanece como un reto clínico importante, sobre todo para la diferenciación entre la masa de conformación pancreatitis crónica, pancreatitis autoinmune y el adenocarcinoma de páncreas. Ecografía endoscópica (USE), la tomografía computarizada (TC) y la resonancia magnética (MRI) pueden proporcionar información valiosa y complementaria en este entorno. Entre ellos, la USE tiene la capacidad única de obtener muestras para diagnóstico histopatológico y por lo tanto, puede desempeñar un papel crucial en la evaluación de los pacientes con resultados poco concluyentes en los exámenes iniciales. Hoy en día, las nuevas técnicas desarrolladas asociadas a la USE, como la elastografía y realce de contraste, han mostrado resultados prometedores para el diagnóstico diferencial de las lesiones pancreáticas.
Palabras clave: Ecoendoscopia. Tomografía computarizada. MRI. Tumores de páncreas.
Introduction
Differential diagnosis of solid pancreatic masses remains a challenge, despite recent advances in different diagnostic procedures. In this article, the role of endoscopic ultrasound (EUS) including EUS-guided FNA, elastography and contrast enhancement will be reviewed in relation to computer tomography (CT), magnetic resonance tomography (MRI) and magnetic resonance cholangiopancreatography (MRCP). Differential diagnosis between malignant lesions and mass-forming chronic pancreatitis or focal autoimmune pancreatitis will be discussed. Tumor staging and evaluation of resectability of these pancreatic lesions will not be evaluated. Other imaging techniques, like PET-scan or octreoscan will not be analyze, because their usefulness in this context is unclear.
Differential diagnosis between malignant pancreatic lesions (pancreatic cancer, pancreatic metastasis) and mass-forming chronic pancreatitis
CT scan is the most widely spread imaging modality for evaluation of pancreatic solid masses and considered as the most comprehensive tool for diagnosis and surgical staging of pancreatic malignancies (1). CT technology has significantly advanced over the last years, multidetector helical CT scan allows for very thin sliced cuts, providing a higher image resolution and faster image acquisition (Fig. 1). Oblique cuts, curved reformation images, and volume 3D reconstructed images can be obtained by post imaging processing. Despite these advances, differential diagnosis between mass-forming chronic pancreatitis, ductal adenocarcinoma, and autoimmune pancreatitis based on only CT image can be challenging (2,3). The vast majority of solid malignancies of the pancreas are hypovascularized and will therefore present low attenuation on contrast enhanced CT. However, isoattenuating pancreatic adenocarcinomas exist, making diagnosis by CT much more difficult. Prokesh et al. reported that 11% of solid pancreatic malignancies were isoattenuating on CT (4). In such cases, secondary signs like dilatation of the pancreatic and/or biliary duct may raise the suspicion of a compressing malignant process. Pancreatic adenocarcinomas area rarely hyper-enhanced (this is mainly associated to neuroendocrine tumors). Differential diagnosis between chronic pancreatitis and pancreatic adenocarcinoma by triple-phase helical CT scan was recently investigated in a study on 42 patients with chronic pancreatitis and 85 patients with pancreatic adenocarcinoma by Yamada et al. (5). protocol with scan delays of 40, 70, and 150 s; and 370 mg I/mL of contrast was used. Mean contrast enhancement in normal pancreatic tissue peaked during the first phase (early-washout pattern) while that of chronic pancreatitis peaked during the second phase (delayed-washout pattern), and that of pancreatic adenocarcinoma gradually increased in all phases. Diagnostic indices for pancreatic adenocarcinoma were 94.1% for sensitivity, 83% for specificity, and 90.4% for accuracy, when differentiation between chronic pancreatitis and pancreatic adenocarcinoma was performed based on time-attenuation curve patterns.
MRI can be useful in the differentiation of pancreatic solid masses but is considered less sensitive than CT and EUS for detection and evaluation of solid pancreatic tumors (6). Pancreatic adenocarcinoma appears hypo intense on T1-weighted images both pre and post contrast injection (Fig. 2). A meta-analysis by Bipat et al. (7), showed MRI to be less sensitive than CT scan (84 vs. 91%) in the detection of pancreatic cancer. The administration of secretin during MRCP can be useful by enhancing the image of the main pancreatic duct, demonstrating dilated side-branches and providing information on pancreatic function (8). Another important advantage of MRI is that it is superior to other imaging modalities in visualizing tumors within areas of pancreatic inflammation (9).
EUS can produce high-resolution images of the pancreas and is considered as one of the most accurate methods for the diagnosis and staging of inflammatory, cystic and neoplastic diseases of the pancreas (10-12) (Fig. 3). Drawbacks include difficulty in differentiation between pancreatic cancer and focal pancreatitis based on B-mode images, particularly in cases of advanced chronic pancreatitis, and the fact that the method is highly operator dependent. Despite the high-resolution images produced by conventional EUS, the accuracy in differentiation between benign inflammatory masses and malignant tumors of the pancreas is not higher than 75% (13-19). This is partly due to the fact that changes like internal and/or peripheral calcifications similar to what is observed in advanced chronic pancreatitis are present in a proportion of pancreatic malignancies. EUS guided fine needle aspiration (EUS-FNA) has the ability to overcome this problem in many cases.
The role of EUS-FNA in the diagnosis of solid pancreatic tumors has been evaluated in several, well-designed studies. Reported sensitivity and accuracy for malignancy ranges from 75 to 92% and from 79 to 92%, respectively (19-33). This accuracy may be even higher using on-site evaluation of the sample by an experienced pathologist (34). Furthermore, in addition to differentiation between benign and malignant lesions, EUS-FNA can also establish the final diagnosis in many cases (33). However, it is important to point out that the sensitivity of EUS-FNA for malignancy in parenchymal masses with features of CP is inferior compared to when the surrounding parenchyma is normal (19,35-39). Fritscher-Ravens et al. (19) found that sensitivity of EUS-FNA in patients with a focal pancreatic lesion without chronic pancreatitis was 89%, while it was only 54% in patients with chronic pancreatitis. Varadarajulu et al. (36) investigated 282 patients with pancreatic solid tumors. Sensitivity of EUS-FNA for malignancy was lower in the group of patients with concurrent chronic pancreatitis compared to those without (73.9 vs. 91.3%; p = 0.02). There were no differences in terms of specificity (100 vs. 93.8%), and overall accuracy (91.5 vs. 91.4%). In a study by Ardengh et al. (37) including 69 pancreatic masses in patients with chronic pancreatitis, EUS-FNA increased diagnostic sensitivity, specificity and overall accuracy in the differential diagnosis between inflammatory process and pancreatic adenocarcinoma (72.7 vs. 63.6%; 100 vs. 75.9%; 95.7 vs. 73.9%; respectively) compared to EUS alone. Takahashi et al. (39) evaluated 62 patients with pancreatic cancer and 15 with focal pancreatitis, reporting sensitivity, specificity, overall accuracy, positive predictive value, and negative predictive value of cytopathology diagnosis of 82, 100, 86, 100, and 58%, respectively. They also observed K-ras point mutations in 74% of pancreatic cancers and 0% of focal pancreatitis lesions.
Certain drawbacks of EUS-FNA need to be emphasized. The procedure is difficult to perform in certain cases, owing to vessel interposition, duodenal stenosis and tumor hardness, particularly in chronic pancreatitis, which hampers the overall accuracy of the procedure. In some occasions EUS-FNA samples cannot be interpreted due to bleeding or non-cellular samples. A systematic review of 53 studies estimated the negative predictive value of EUS-FNA in the diagnosis of pancreatic adenocarcinoma to 60-70% (40). Hence, a new puncture seems mandatory in order to exclude malignancy in cases where the first EUS-FNA has been benign (41). Given the high accuracy in the evaluation of pancreatic tumors, Eloubeidi et al. concluded in a recent study that routine EUS-FNA for the differential diagnosis of solid pancreatic masses can be recommended (42).
In order to optimize tissue retrieval of EUS-FNA, various EUS-guided techniques have been explored, including FNA and Tru-Cut needles, with variable success and complication rates (43-46). Of particular interest is the Quick-Core® needle, designed to operate through an echoendoscope. EUS-guided use of Quick-Core® needle has demonstrated that histological samples representative of the target organs can be obtained safely (47,48). However, there are certain drawbacks with the Quick-Core® needle that restrict its use in clinical practice. Most importantly, its diagnostic yield is strongly limited for lesions located in pancreatic head due to mechanical friction of the needle firing mechanism ensuing from the bended scope position (49-52). In this setting a novel needle have been designed (Procore™ histology needle) to overcome trucut needle limitations (mainly in the second portion of the duodenum) and initial preliminary results are promising and it appears that may help solve this problem, allowing a histological evaluation with an overall accuracy of 85.9% (89.4% in pancreatic solid lesions) (53). There are also other needles in the market (Olympus EZShot2 NA-230H-8022), designed to obtain histological samples, but its clinical usefulness and safety are still under evaluation.
EUS elastography is a noninvasive technique that measures elasticity in real time by registration of differences in distortion of the EUS image after application of slight pressure by the EUS probe (Figs. 4 and 5). Many different pathological processes, including inflammation, fibrosis and cancer, can alter tissue elasticity that will result in distinct elastographic appearance. The first studies published on EUS elastography were based on qualitative elastography evaluation, using a hue-color scale representing different degrees of tissue elasticity. Giovannini et al. (54) analyzed 24 pancreatic masses using a scoring system based on different color patterns in EUS elastography images. Sensitivity and a specificity of 100 and 67% respectively were observed in the differentiation between benign and malignant pancreatic masses. In a multicenter study (55), including 121 pancreatic masses, sensitivity and specificity of EUS elastography to differentiate benign from malignant pancreatic lesions were 92.3 and 80.0%, respectively, compared to 92.3 and 68.9%, respectively, for the conventional B-mode images. The interobserver agreement was substantial (kappa score = 0.785). Another paper published by Iglesias-García et al. (56), analyzed 130 patients with solid pancreatic masses. Malignancy could be diagnosed by qualitative EUS-elastography using color patterns with a sensitivity, specificity, positive and negative predictive values, and overall accuracy of 100, 85.5, 90.7, 100 and 94.0%, respectively. However, substantially lower diagnostic performance by qualitative elastography has been reported. Hirsche et al. (57), presented their results on 70 patients with unclassified solid pancreatic lesions, and found that they could only perform an adequate evaluation in 56% of patients, and that EUS-elastography predicted the nature of pancreatic lesions with poor diagnostic sensitivity (41%), specificity (53%) and accuracy (45%). Recently quantitative EUS elastography has been developed in an attempt to make the elastography interpretation less subjective. Quantitative elastography renders a numeric result, either as mean value of hues in a selected area (mean hue histogram) or as a ratio of elasticity in the target area over soft reference tissue (strain ratio). Iglesias-García et al. (14), have evaluated strain ratio in 86 consecutive patients with solid pancreatic masses (49 adenocarcinomas, 27 inflammatory masses, 6 malignant neuroendocrine tumors, 2 metastatic oat cell lung cancers, 1 pancreatic lymphoma, and 1 pancreatic solid pseudopapillary tumor) and 20 controls. The strain ratio was significantly higher among patients with malignant pancreatic tumors compared to those with inflammatory masses. Normal pancreatic tissue showed a mean strain ratio of 1.68 (95%CI: 1.59-1.78). Inflammatory masses presented a strain ratio (mean 3.28; 95%CI: 2.61-3.96) significantly higher than that of the normal pancreas (p < 0.001), but lower than that of pancreatic adenocarcinoma (mean 18.12; 95%CI: 16.03-20.21) (p < 0.001). The highest strain ratio was found among endocrine tumors (mean 52.34; 95%CI: 33.96-70.71). The sensitivity and specificity of the strain ratio for detecting pancreatic malignancies using a cut-off value of 6.04 were 100% and 92.9%, respectively, exceeding the accuracy obtained with qualitative elastography. Saftoiu et al. (58) evaluated the usefulness of the hue-histograms in a similar setting. Based on a cutoff of 175 for the mean hue histogram value, the sensitivity, specificity, and accuracy of differentiation of benign and malignant masses were 91.4, 87.9, and 89.7%, respectively. The positive and negative predictive values were 88.9 and 90.6%, respectively. The same methodology and cut-off value was used in a subsequent multicenter study including 258 patients. A sensitivity of 93.4%, a specificity of 66.0%, a positive predictive value of 92.5%, a negative predictive value of 68.9%, and an overall accuracy of 85.4% for the mean hue-histogram in the detection of malignancy were observed (59).
Administration of contrast agents is another way to improve EUS based diagnosis of solid pancreatic tumors. Initial studies using Levovist®, Albunex and FS 069 Optison as contrast agents indicated that contrast enhanced EUS could be useful to detect malignant vascular infiltration and demonstrated that the hyper vascular aspect of neuroendocrine tumors and the hypo vascular aspect of pancreatic adenocarcinoma could be readily demonstrated (60-64). Modern contrast enhanced EUS relies on a dedicated contrast harmonic echo (CHE-EUS) technique that detects signals from micro bubbles delivered by new contrast agents like Sonovue® in vessels with very slow flow without the burden of Doppler-related artifacts. Fusaroli et al. investigated 90 patients with solid pancreatic lesions by CEH-EUS, using Sonovue® as contrast agent (65). The finding of a hypo-enhancing mass with an inhomogeneous pattern diagnosed pancreatic adenocarcinoma with a sensitivity of 96% and an accuracy of 82%. The study also indicated that this CEH-EUS pattern diagnosed malignancy more accurately than the finding of a hypoechoic mass on standard EUS (p < 0.0001). Hyper-enhancement specifically excluded adenocarcinoma (98%), although sensitivity was low (39%). An interesting observation was that CHE-EUS detected small lesions in 7 patients where conventional EUS findings were inconclusive due to biliary stents or chronic pancreatitis. In a recent study by Napoleon et al. (66) on 35 patients with solid pancreatic lesions, the finding of a hypo-enhanced lesion was able to detect malignancy with a sensitivity, specificity and accuracy of 89, 88, and 88.5%, respectively. Four out of five patients with adenocarcinomas with false-negative results at EUS-FNA had a hypo-enhanced appearance on CEH-EUS in this series. Seicean et al. (67) investigated the possibility to use quantitative CEH-EUS data in the differential diagnosis between pancreatic cancer and chronic pancreatitis. A hypo-enhanced pattern was the most common finding both in with pancreatic adenocarcinoma (14/15 cases) and in mass forming chronic pancreatitis (10/12 cases). However, an index of contrast uptake ratio was calculated and this was significantly lower in adenocarcinoma compared to cases with mass-forming chronic pancreatitis. A cut-off uptake ratio index value of 0.17 for diagnosing adenocarcinoma corresponded to an AUC of 0.86 (95%CI: 0.67-1.00) with a sensitivity of 80%, a specificity of 91.7%, a positive predictive value of 92.8%, and a negative predictive value of 78%. Generally, differences in histology, such as histological differentiation grade, amount of fibrosis and obliteration of blood vessels in the tumor, may be associated with differences in enhancement behavior.
Differential diagnosis of focal autoimmune pancreatitis
Autoimmune pancreatitis is a recently described but today globally acknowledged entity (68-71). The clinical presentation of autoimmune pancreatitis may be very similar to pancreatic cancer (72-74). Autoimmune pancreatitis has been demonstrated in 3 to 5% of specimens from patients undergoing surgical resection for suspected pancreatic (75). There is no single test that can reliably diagnose autoimmune pancreatitis. The diagnosis is based on a combined of pancreatic histology, imaging, serology, other organ involvement, and response to steroids (76-80).
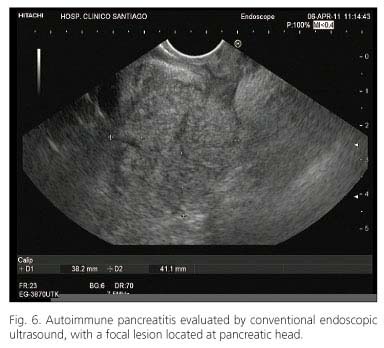
Findings on CT scan or MRI are often the first clues that raise the suspicion of pancreatic cancer or autoimmune pancreatitis. Typical findings on CT suggestive for autoimmune pancreatitis include focal or diffusely enlarged pancreas without dilatation of the main pancreatic duct, a capsule-like rim around the pancreas and the absence of calcifications and pseudocysts (Fig. 6). On the other hand, a low-density mass on contrast-enhanced CT with pancreatic ductal dilatation or stricture are indicative for pancreatic cancer. Diagnostic work-up in unclear cases should primarily be directed at excluding pancreatic cancer (80).
Autoimmune pancreatitis is associated with elevated titers of IgG, IgG4, and antinuclear antibody (81,82). However, false positive elevations have been observed in all of these serological markers in patients with other pancreatic disorders, including pancreatic cancer. Gahzaale et al. have investigated the role of serum IgG4 measurement in a study on 510 patients (45 with autoimmune pancreatitis, 135 with pancreatic cancer, 62 without pancreatic disease and 268 with other pancreatic diseases). A sensitivity, specificity and positive predictive value of 76, 93 and 36% for the diagnosis of autoimmune pancreatitis was found when the upper normal limit (140 mg/dL) was used as cutoff value. Corresponding diagnostic performance values were 53, 99 and 75%, respectively, if the cutoff value was elevated to two times the upper normal limit (280 mg/dL) (77). Autoimmune pancreatitis is a systemic disease and involvement of other organs is another finding that can support the diagnosis. Other organ involvement may be diagnosed by histology, imaging (proximal bile duct stricture, retroperitoneal fibrosis) or clinical examination (salivary gland enlargement). EUS-guided biopsies using large core biopsy needles can be used to obtain histological specimens for the diagnosis of autoimmune pancreatitis. Demonstration of the typical lymphoplasmacytic infiltrate has been proven to be possible and safe with EUS guided biopsies using the Tru-Cut needle (Quick-Core®) (83,84). However, histological diagnosis of autoimmune pancreatitis is extremely difficult (even using Tru-cut or Procore™ needle) and diagnosis with EUS-FNA cytology needles is almost impossible. Response to steroid treatment is a final criterion that can be used to differentiate between autoimmune pancreatitis and pancreatic cancer. A treatment trial is usually performed by oral administration of prednisone 0.5 mg/kg during two weeks followed by reassessment of imaging and CA19-9 (85). An improvement of imaging abnormalities, including biliary strictures and pancreatic enlargement is required for confirmation of the diagnosis of autoimmune pancreatitis. Moon et al. (85) studied the effect of steroid challenge in 22 patients with a clinical suspicion of autoimmune pancreatitis but with imaging findings that could not conclusively differentiate between pancreatic cancer and autoimmune pancreatitis. Steroid treatment led to radiological improvement in all patients with a final diagnosis of autoimmune pancreatitis (n = 15) and no response in patients where the final diagnosis was pancreatic cancer (n = 7). International consensus guidelines for the diagnosis of autoimmune pancreatitis have been recently published by the International Association of Pancreatology (80).
Conclusions
EUS, CT and MRI can all provide valuable and complementary information in the differential diagnosis between mass forming chronic pancreatitis, autoimmune pancreatitis and pancreatic adenocarcinoma. EUS has the unique ability to obtain specimens for histopathological diagnosis and can therefore play a crucial role in the evaluation patients with inconclusive findings on initial examinations. EUS guided elastography and the uses of contrast agents together with harmonic echo are also useful in this setting.
References
1. Kinney T. Evidence-based imaging of pancreatic malignancies. Surg Clin North Am 2010;90:235-49. [ Links ]
2. Taylor B. Carcinoma of the head of the pancreas versus chronic pancreatitis: diagnostic dilemma with significant consequences. World J Surg 2003;27:1249-57. [ Links ]
3. Frulloni L, Falconi M, Gabrielli A, Gaia E, Graziani R, Pezzilli R, et al. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis 2010;42:S381-406. [ Links ]
4. Prokesh RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB Jr. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology 2002;224:764-8. [ Links ]
5. Yamada Y, Mori H, Matsumoto S, Kiyosue H, Hori Y, Hongo N. Pancreatic adenocarcinoma vs chronic pancreatitis: differentiation with triple phase helical CT. Abdom Imaging 2010;35:163-71. [ Links ]
6. Hakimé A, Giraud M, Vullierme MP, Vilgrain V. MRI imaging of the pancreas. J Radiol 2007;88:11-25. [ Links ]
7. Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS, et al. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determing resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assit Tomogr 2005;29:438-45. [ Links ]
8. Sandrasegaran K, Lin C, Akisik FM, Tann M. State-of-the-art pancreatic MRI. AJR Am J Roentgenol 2010;195(1):42-53. [ Links ]
9. Meng Z, Xu YK, Zhang YP. Magnetic resonance cholangiopancreatography of pancreaticobiliary duct dilation due to pancreatic carcinoma and chronic pancreatitis. Nan Fang Yi Ke Da Xue Xue Bao 2008;28:113-5. [ Links ]
10. Seicean A. Endoscopic ultrasound in chronic pancreatitis: where are we now? World J Gastroenterol 2010;16:4253-63. [ Links ]
11. Giovannini M. The place of endoscopic ultrasound in bilio-pancreatic pathology. Gastroenterol Clin Biol 2010;34:436-45. [ Links ]
12. Varadajulu S, Eloubeidi MA. The role of endoscopic ultrasonography in the evaluation of pancreatico-biliary cancer. Surg Clin North Am 2010;90:251-63. [ Links ]
13. Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol 2000;97(6):1386-91. [ Links ]
14. Iglesias-García J, Lariño-Noia J, Abdulkader I, Forteza J, Domínguez-Muñoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterol 2010;139 (4):1172-80. [ Links ]
15. Byrne MF, Jowell PS. Gastrointestinal imaging: endoscopic ultrasound. Gastroenterology 2002;122:1631-48. [ Links ]
16. Hawes RH, Zaidi S. Endoscopic ultrasonography of the pancreas. Gastrointest Endosc Clin N Am 1995;5:61-80. [ Links ]
17. Wallace MB, Hawes RH, Durkalski V, Chak A, Mallery S, Catalano MF, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001;53:294-9. [ Links ]
18. Kaufman AR, Sivak MV. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. Gastrointest Endosc 1989;35:214-9. [ Links ]
19. Fritscher-Ravens A, Brand L, Knöfel WT, Bobrowski C, Topalidis T, Thonke F, et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol 2002;97: 2768-75. [ Links ]
20. Giovannini M, Seitz JF, Monges F, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy 1995;27:171-7. [ Links ]
21. Bhutani MS, Hawes RH, Baron PL, Sanders-Cliette A, van Velse A, Osborne JF, et al. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy 1997;29:854-8. [ Links ]
22. Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Lehman GA. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc 1997;45:243-50. [ Links ]
23. Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc 1997;45:387-93. [ Links ]
24. Faigel DO, Ginsberg GG, Bentz JS, Gupta PK, Smith DB, Kochman ML. Endoscopic ultraosound-guided real-time fine-needle aspiration biopsy of the pancreas in cancer patients with pancreatic lesions. J Clin Oncol 1997;15:1439-43. [ Links ]
25. Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assesement. Gastroenterology 1997;112:1087-95. [ Links ]
26. Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Web J. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single center experience. Gut 1999;44:720-6. [ Links ]
27. Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O'Toole D. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut 2000;46:244-9. [ Links ]
28. Gress F, Gottlieb K, Sherman S, Lehman G. Endoscopic ultrasonography-guided fine needle aspiration biopsy of suspected pancreatic cancer. Ann Inter Med 2001;134: 459-64. [ Links ]
29. Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol 2002;97:1386-91. [ Links ]
30. Raut CP, Grau AM, Staerkel GA, Kaw M, Tamm EP, Wolff RA. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg 2003;7:118-26. [ Links ]
31. Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-days complications. Am J Gastroenterol 2003;98(12):2663-8. [ Links ]
32. Ardengh JC, Lopes CV, Pereira de Lima LP, Rodrigues de Oliveira J, Venco F, Santo GC, et al. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol 2007;13(22):3112-6. [ Links ]
33. Iglesias-García J, Domínguez-Muñoz JE, Lozano-León A, Abdulkader I, Lariño-Noia J, Antunez J, et al. Impact of endoscopic-ultrasound fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol 2007;13(2):289-93. [ Links ]
34. Iglesias-García J, Domínguez-Muñoz JE, Abdulkader I, Lariño-Noia J, Eugenyeva E, Lozano-León A, et al. Influence of On-Site Cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound guided fine needle aspiration of solid pancreatic masses. Am J Gastroenterol 2011;106(9):1705-10. [ Links ]
35. Barthet M, Portal I, Boujaoude J, Bernard JP, Sahel J. Endoscopic ultrasonographic diagnosis of pancreatic cancer complicating chronic pancreatitis. Endoscopy 1996;28:487-91. [ Links ]
36. Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUSguided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc 2005;62:728-36. [ Links ]
37. Ardengh JC, Lopes CV, Campos AD, Pereira de Lima LF, Venco F, Módena JL. Endoscopic ultrasound and fine needle aspiration in chronic pancreatitis: differential diagnosis between pseudotumoral masses and pancreatic cancer. JOP 2007;8:413-421 54. [ Links ]
38. Krishna NB, Mehra M, Reddy AV, Agarwal B. EUS/EUSFNA for suspected pancreatic cancer: influence of chronic pancreatitis and clinical presentation with or without obstructive jaundice on performance characteristics. Gastrointest Endosc 2009;70:70-9. [ Links ]
39. Takahashi K, Yamao K, Okubo K, Sawaki A, Mizuno N, Ashida R, et al. Differential diagnosis of pancreatic cancer and focal pancreatitis by using EUS-guided FNA. Gastrointest Endosc 2005;61:76-9. [ Links ]
40. Hartwig W, Schneider L, Diener MK, Bergmann F, Büchler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg 2009;96:5-20. [ Links ]
41. Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol 2008;23:567-70. [ Links ]
42. Eloubeidi MA, Varadarajulu S, Desai S, Shirley R, Heslin MJ, Mehra M, et al. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine needle aspiration in suspected pancreatic cancer. J Gastrointest Surg 2007;11(7):813-9. [ Links ]
43. Binmoeller KF, Thul R, Rathod V, Henke P, Brand B, Jabusch HC, et al. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc 1998;47:121-7. [ Links ]
44. Harada N, Kouzu T, Arima M, Isono K. Endoscopic ultrasound-guided histologic needle biopsy: preliminary results using a newly developed endoscopic ultrasound transducer. Gastrointest Endosc 1996;44:327-30. [ Links ]
45. Wiersema MJ, Levy MJ, Harewood GC, Vazquez-Sequeiros E, Jondal ML, Wiersema LM. Initial experience with EUS-guided Trucut needle biopsy of perigastric organs. Gastrointest Endosc 2002;56:275-8. [ Links ]
46. Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc 2003;57:101-6. [ Links ]
47. Levy MJ, Wiersema MJ. EUS-guided Trucut Biopsy. Gastrointest Endosc 2005;62:417-26. [ Links ]
48. Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Best Practice & Research Clinical Gastroenterology 2009;23:743-59. [ Links ]
49. Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc 2004;59: 185-90. [ Links ]
50. Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, et al. Comparison of EUS guided 19-gauge Trucut needle biopsy with EUS-guided fine needle aspiration. Endoscopy 2004;36:397-401. [ Links ]
51. Wahnschaffe U, Ullrich R, Mayerle J, Lerch MM, Zeitz M, Faiss S. EUS-guided Trucut needle biopsies as first-line diagnostic method for patients with intestinal or extraintestinal mass lesions. Surg Endosc 2009;23:2351-5. [ Links ]
52. Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety and predictive factors for a positive yield of EUS-Guided trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol 2009;104:584-91. [ Links ]
53. Iglesias-García J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc 2011;73(6):1189-96. [ Links ]
54. Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic Ultrasound Elastography: the First Step towards Virtual Biopsy? Preliminary Results in 49 patients. Endoscopy 2006;38:344-8. [ Links ]
55. Giovannini M, Botelberge T, Bories E, Pesenti C, Caillol F, Esterni B, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: A multicenter study. World J Gastroenterol 2009;15(13):1587-93. [ Links ]
56. Iglesias-García J, Lariño-Noia J, Abdulkader I, Forteza J, Domínguez-Muñoz JE. EUS-elastography for the characterization of solid pancreatic masses. Gastrointest Endosc 2009;70(6):1101-8. [ Links ]
57. Hirche TO, Ignee A, Barreiros AP, Schreiber-Dietrich D, Jungblut S, Ott M, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy 2008;40(11):910-7. [ Links ]
58. Saftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, et al. Neural network analysis of dynamic sequences of EUS elastography used for differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc 2008;68(6):1086-94. [ Links ]
59. Saftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy 2011;43(7):596-603. [ Links ]
60. Bhutani MS, Hoffman BJ, van Velse A, Hawes RH. Contrast-enhanced endoscopic ultrasonography with galactose microparticles: SHU508 A (Levovist). Endoscopy 1997;29(7):635-9. [ Links ]
61. Hirooka Y, Goto H, Ito A, Hayakawa S, Watanabe Y, Ishiguro Y, et al. Contrast-enhanced endoscopic ultrasonography in pancreatic diseases: a preliminary study. Am J Gastroenterol 1998;93(4):632-5. [ Links ]
62. Becker D, Strobel D, Bernatik T, Hahn EG. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc 2001;53(7):784-9. [ Links ]
63. Giovannini M. Endosonography: new developments in 2006. ScientificWorldJournal 2007;7:341-63. [ Links ]
64. Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol 2006;12: 246-50. [ Links ]
65. Fusaroli P, Spada A, Mancino MG. Contrast Harmonic Echo-Endoscopic Ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clinical Gastroenterol Hepatol 2010;8:629-34. [ Links ]
66. Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy 2010;42:564-70. [ Links ]
67. Seicean A, Badea R, Stan-Iuga R, Mocan T, Gulei I, Pascu O. Quantitative contrast-enhanced harmonic Endoscopic Ultrasound for the discrimination of solid pancreatic masses. Ultraschall Med 2010;31:571-6. [ Links ]
68. Sarles H, Sarles JC, Camatte R, Muratore R, Gaini M, Guien C, et al. Observations on 205 confirmed cases of acute pancreatitis, recurring pancreatitis, and chronic pancreatitis. Gut 1965;6:545-59. [ Links ]
69. Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol 1991;22:387-95. [ Links ]
70. Sugumar A, Kloppel G, Chari ST. Autoimmune pancreatitis: pathologic subtypes and their implications for its diagnosis. Am J Gastroenterol 2009;104:2308-10. [ Links ]
71. Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci 1995; 40:1561-8. [ Links ]
72. Chutaputti A, Burrell MI, Boyer JL. Pseudotumor of the pancreas associated with retroperitoneal fibrosis: a dramatic response to corticosteroid therapy. Am J Gastroenterol 1995;90:1155-8. [ Links ]
73. Hayakawa T, Naruse S, Kitagawa M, Kondo T. Clinical aspects of autoimmune pancreatitis in Sjögren's syndrome. JOP 2001;2:88-92. [ Links ]
74. Lara LP, Chari ST. Autoimmune pancreatitis. Curr Gastroenterol Rep 2005;7:101-6. [ Links ]
75. Wolfson D, Barkin JS, Chari ST, Clain JE, Bell RH Jr, Alexakis N, et al. Management of pancreatic masses. Pancreas 2005;31:203-17. [ Links ]
76. Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 2006;4:1010-6. [ Links ]
77. Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 2007;102:1646-53. [ Links ]
78. Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732-8. [ Links ]
79. Japan Pancreas Society: Diagnostic criteria for autoimmune pancreatitis. J Jpn Pancreas Soc 2002;17:585-7. [ Links ]
80. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International Consensus Diagnostic Criteria for Autoimmune Pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011;40:352-8. [ Links ]
81. Sadler R, Chapman RW, Simpson D, Soonawalla ZF, Waldegrave EL, Burden JM, et al. The diagnostic significance of serum IgG4 levels in patients with autoimmune pancreatitis: a UK study. Eur J Gastroenterol Hepatol 2011;23:139-45. [ Links ]
82. Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M, et al. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol 2011;46:277-88. [ Links ]
83. Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol 2009;44:742-50. [ Links ]
84. Levy MJ, Reddy RP, Wiersema MJ, Smyrk TC, Clain JE, Harewood GC, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointes Endosc 2005;61:467-72. [ Links ]
85. Moon S-H, Kim MH, Park DH, Hwang CY, Park SJ, Lee SS, et al. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut 2008;57:1704-12. [ Links ]
![]() Correspondence:
Correspondence:
Julio Iglesias-García
Department of Gastroenterology
Foundation for Research in Digestive Diseases (FIENAD). of Santiago de Compostela
Choupana, s/n 15706
Santiago de Compostela. A Coruña
e-mail: julio.iglesias.garcia@sergas.es
Received: 27-03-2012
Accepted: 20-04-2012