Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.105 no.9 Madrid oct. 2013
https://dx.doi.org/10.4321/S1130-01082013000900009
Sclerosing cholangitis by cytomegalovirus in highly active antiretroviral therapy era
Colangitis esclerosante por citomegalovirus en la era del tratamiento antirretroviral de alta eficacia
Carmen Hidalgo-Tenorio and Gonzalo Blasco-Morente
Infectious Diseases Unit. Hospital Universitario Virgen de las Nieves. Granada, Spain
ABSTRACT
Sclerosing colangitis (SC) due to cytomegalovirus (CMV) is very rare. It has been described mainly in immunocompromised patients. Currently, in HIV infected patients it is exceptional. The most of cases belong to pre-highly active antiretroviral therapy (pre-HAART) and those cases were in stage AIDS with less than 100 CD4/μl. The most frequently involved pathogen in pre-HAART period was Cryptosporidium parvum (30-57%) and CMV (10-30%); in late HAART period this information are unaware. CMV has been implicated as a possible etiological agent in primary SC partly because of the ability to cause liver damage and its relationship with smooth muscle antibodies. The most effective treatment for SC was the combination of antiretroviral therapy and endoscopic retrograde cholangiopancreatography with sphincterotomy and stent placement.
Following, we present the first case of late HAART period which describes a SC extrahepatic without papillary stenosis with CMV as the only cause and clinical presentation of HIV infection in a woman with 177 CD4/μl.
Key words: AIDS. Cytomegalovirus. Sclerosing cholangitis. Opportunistic infections.
RESUMEN
La colangitis esclerosante (CE) debida a infección por citomegalovirus (CMV) es muy rara; se ha descrito principalmente en inmunodeprimidos. En pacientes infectados por VIH es actualmente excepcional y la mayoría de casos fueron comunicados durante el periodo previo al de la terapia antirretroviral de alta eficacia (pre-TAR), y se encontraban en estadio sida con cifras de CD4 inferiores a los 100 cél/μl. En la era pre-TAR el organismo más frecuentemente implicado era Cryptosporidium parvum (30-57%) y CMV (10-30%); del periodo TAR se desconocen estos datos. El CMV ha sido implicado como un posible agente etiológico de CE primaria por su capacidad para producir daño hepático y su relación con anticuerpos antimúsculo liso. El tratamiento que ha demostrado mayor eficacia ha sido la combinación de antirretrovirales y realización de colangiopancreatografía retrógrada endoscópica con esfinterotomía y colocación de endoprótesis.
A continuación presentamos el primer caso en la era tardía (a partir de 2005) del tratamiento antirretroviral de CE extrahepática sin estenosis papilar por CMV como causa única y como forma clínica de presentación de infección por VIH en una mujer con 177 CD4/μl.
Palabras clave: Sida. Citomegalovirus. Colangitis esclerosante. Infecciones oportunistas.
Introduction
Sclerosing cholangitis (SC) due to cytomegalovirus (CMV) is very rare, and has been described mainly in immunocompromised patients: Liver and renal transplant recipients, X-linked hyperimmunoglobulin E syndrome, with chronic glucocorticoids and HIV patients in the AIDS stage (1-4); and finally, in just one immunocompetent case (5).
In HIV-infected patients, it is exceptionally rare, which has been noted mainly in the pre-highly active antiretroviral therapy (pre-ART) period, with most patients at the AIDS stage and with a CD4 cell count below 100 cells/μl (1,2,6-8). AIDS-associated cholangiopathy has been defined as an obstructive syndrome that is produced as a result of infection of biliary tract structures. Prior to the emergence of highly active antiretroviral therapy (HAART), cholangiopathy came to affect one in four AIDS patients (9). Currently in the era of ART, there is no data about the incidence of this disease, that being CMV gastrointestinal involvement, occurring in 4.7% of HIV patients and including illnesses ranging from colitis, esophagitis, gastritis, hepatitis to sclerosing cholangitis (10). Subsequently, we describe a woman recently diagnosed with HIV and CMV sclerosing cholangitis simultaneously, whose baseline CD4 cell count was 177 cells/μl, and who was successfully treated with antiviral, antiretroviral drugs, and endoscopic retrograde cholangiopancreatography (ERCP).
Case report
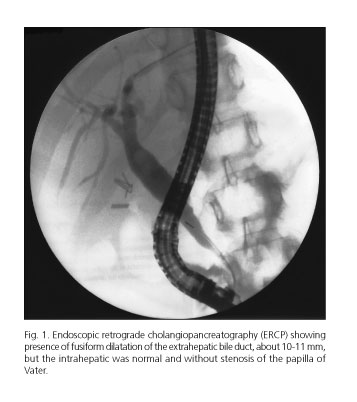
A 35-year-old woman from Morocco with a history of cholecystectomy, no toxic habits or sexual risk factors was hospitalised in May 2011 for having fevers as high as 38.5 oC. She was suffering from colic pain in her right hypochondrium and epigastrium, as well as from pruritus and dysphagia. A physical examination revealed tenderness in her abdomen, located particularly in her upper right hypochondrium, although there were no signs of peritoneal irritation, jaundice, or oral thrush. The rest of the history and physical examination were normal. The laboratory analysis showed the following: 10.9 g/dL hemoglobin, 82 fL MCV, 2300 WBC/μL, 58.3% neutrophils, 0.1 mg/dL C-reactive protein, 1.44 mg/dL total bilirubin (TBIL), 663 U/L alkaline phosphatase (ALP), 185 U/L γ-glutamyl transpeptidase (GGT), 231 U/L aspartate transaminase (AST), 156 U/L alanine transaminase (ALT), 2.04 g/dL immunoglobulin (Ig), 553 mg/dL IgA, 2,400 mg/dL IgG and 62 mg/dL IgM; a positive atypical pattern 1:320 U/L of perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA), and a positive 1:320 U/L of smooth anti-muscle (SMA). Antineutrophil cytoplasmic (c-ANCA), antinuclear (ANA), antimitochondrial (AMA), antimicrosomal (LKM-1) and antisaccharomyces antibodies (ASCA) were all negative. The serology for HIV was positive, and the viral load was greater than 10 million copies/mL, the CMV IgG was positive and the viral load was 2-5.000.000 copies/ml, Rubella IgG was positive and Toxoplasma gondii IgG was greater than 250 IU/mL. Whilst hepatitis A, B and C, syphilis, Brucella spp, Cryptococcus, Leishmania spp., and CMV IgM were all negative; EBV IgM showed indeterminate results. The following tests were negative: Giardia lamblia antigen and fecal Cryptosporidium, the Legionella and pneumococcal urinary antigen, the smears and cultures of sputum, the blood and urine cultures. TST were negative. The lymphocyte populations were 177 CD4/μL, 534 CD3/μL, 346 CD8/μL and a CD4/CD8 ratio of 0.51. Indirect ophthalmoscopy was normal. The abdominal ultrasound showed a fusiform dilation of common bile duct, and digestive endoscopy showed white plaques all around the esophagus, which is suggestive of esophageal candidiasis. A cholangiography was carried out which found a dilatation of the intrahepatic bile duct with a stenotic segment and hepatocholedochus with slight dilatation but without stenosis or stones. When the patient was admitted, they received medical treatment including 500 mg of ganciclovir intravenously every 12 hours for two weeks, followed by 900 mg of valganciclovir every 24 hours. As of the second week, antiretroviral (emtricitabine/tenofovir, darunavir and ritonavir) treatment was introduced. Alongside this, the patient was prescribed 100 mg of fluconazole to be taken orally every 24 hours for 7 days, 300 mg of ursodeoxycholic acid to be taken every 12 hours, 4 g of cholestyramine to be taken every 12 hours, 6 mg of dexchlorpheniramine to be taken every 8 hours, and 800/160 mg of cotrimoxazole to be taken three times a week. Despite medical treatment the patient continued with cholestasis (10.26 mg/dL TBIL, 544 U/L ALP, 175 U/L GGT, 448 U/L AST, and 170 U/L ALT) which was the reason for carrying out an endoscopic retrograde cholangiopancreatography (ERCP) 25 days after initiating treatment with ganciclovir. The ERCP confirmed the presence of fusiform dilatation of the extrahepatic bile duct, but the intrahepatic was normal, allowing for treatment involving a sphincterotomy, by placing a stent in the extrahepatic area (Fig. 1). Four days after the ERCP the patient improved as much clinically as analytically, 310 U/L ALP, 94 U/L GGT, 212 U/L AST, 84 U/L GPT, negative CMV viral load and 1,602 copies/mL of HIV). In subsequent revisions, the patient maintained a stable condition with persistent elevation of ALP ranging from 400-600 U/L, and a negative HIV and CMV viral load.
Discussion
The majority of cases of AIDS with sclerosing cholangitis were reported between 1995 and 1996, before the introduction of ART. In approximately 20% of those with SC no germs were identified; when patients did develop SC, Cryptosporidium parvum (30-57%) was the most frequent infection, followed by CMV (10-30%), and coinfection with both (6-20%); other pathogens described were Microsporidia, Cyclospora cayetanensis, Isospora, Giardia and Mycobacterium avium intracellulare (1,2). Following the introduction of ART, there was a significant reduction in the incidence of AIDS-related infections, including CMV infection (10). In the early stages when ART first emerged, which dates to as late as 2005, only twelve cases had been described in which microbiological isolation was achieved in 83.3% of cases, and where Microsporidia, Cryptosporidium and Isospora belli were the only 3 isolated microorganisms (6). At a later stage with ART (> 2005) only two cases have been reported; one in which CMV was implicated as the sole cause of duodenal papillary stenosis, and another in which coinfection with CMV and Cryptosporidium were identified (7,8).
The diagnosis here is based on indistinguishable cholangiographic findings from primary SC, but has no relation to inflammatory bowel disease. Four cholangiographic patterns have been described: SC and papillary stenosis (50-60%), intra-and extrahepatic SC without papillary stenosis (20%), papillary stenosis alone (10%), and extensive involvement of extrahepatic bile duct with or without intrahepatic SC (2,6). CMV as an etiologic agent of this disease has been found in less than one third of cases where germs have been identified, less frequently as the sole cause, and in patients with severe cellular immunosuppression under 50 CD4/μL (8,10). All this makes our case unique given that it is the first, in the later ART era, which describes extrahepatic SC without duodenal papillary stenosis and with CMV as the sole cause, as well as with a CD4 count of 177 cells/μL. Some studies implicate CMV as being a possible etiologic cause of primary SC due to the fact that this virus can cause liver damage, and furthermore, genetic studies have detected CMV DNA in livers of those affected by this disease. An autoimmune etiology for AIDS associated SC similar to primary SC can support this hypothesis. In this field, positive anti-SMA antibodies are described in 17.3% of CMV infected liver transplant recipients (11), and in 16% of blood donors with CMV IgM antibodies or serum IgG (12). The p-ANCA with an atypical pattern has more specificity for bactericidal/permeability-increasing protein and is mainly associated with chronic infections such as HIV, HCV, Parvovirus B19, subacute endocarditis due to Staphylococcus aureus or Streptococcus spp., tuberculosis, leprosy, malaria, amebiasis, aspergillosis, histoplasmosis, leptospirosis, and pulmonary sporotrichosis; the aforementioned has also been associated with post-infectious glomerulonephritis, drugs, connective tissue diseases, gastrointestinal disorders, cancer and environmental factors (13,14). Our patient showed positive anti SMA and p-ANCA which we believe was a result of co-infection with HIV and CMV.
Although infection is the most common cause of AIDS-associated SC, medical treatment for the causative microorganism does not improve clinical or abnormalities of the bile ducts, although its use is still recommended (2,6). The treatment that has proven most effective is a combination of ART and an ERCP alongside a sphincterotomy and stenting (2,6). ERCP has resulted in pain relief and a reduction of biliary obstruction. ART enhances the immune system and is the most effective medical treatment to control opportunistic infections. The similarity to primary SC allows for the use of ursodeoxycholic acid in an experiment, observing a small group of patients show an improvement in symptoms and a decrease in their ALP and GGT levels (15).
The prognosis for those patients in the pre-ART stage was not affected by cholangiopathy, but by the natural history of HIV, with age being a protective factor, contrary to what happens in primary SC (1); currently this information is unknown. Our case is interesting because it is the first case reported in the late ART period in which an extrahepatic SC is described without duodenal papillary stenosis having CMV as the sole cause. The reasons behind why we believe it is worthwhile publishing the findings from this particular case study are firstly the rarity, nowadays, of AIDS-associated cholangiopathy, in addition to the low frequency with which this herpes virus is the causative agent of this disease. Furthermore, our case is a patient with CD4 values three times above normal figures, and demonstrates the reaction to a combination of medical and interventional treatment. Finally, we must stress the importance of early diagnosis of HIV, and it is vital to use the hospital as another means of performing serology on every subject that has not been previously requested, whether risk factors for acquisition are acknowledged or not.
References
1. Forbes A, Blanshard C, Gazzard B. Natural history of AIDS related sclerosing cholangitis: A study of 20 cases. Gut 1993;34:116-21. [ Links ]
2. Benhamou Y, Caumes E, Gerosa Y, Cadranel JF, Dohin E, Katlama C, et al. AIDS-related cholangiopathy. Critical analysis of a prospective series of 26 patients. Dig Dis Sci 1993;38:1113-8. [ Links ]
3. Tiple A, Kamar N, Esposito L, Mengelle C, Combelles S, Otal P, et al. Unusual presentation of cytomegalovirus infection in patients after organ transplant. Exp Clin Transplant 2009;7:45-9. [ Links ]
4. Watanabe T, Joko K, Yokota T, Kobayashi Y, Oono Y, Takechi S, et al. Pancreatitis and cholangitis due to cytomegalovirus in a patient with hyperimmunoglobulin E syndrome. Pancreas 2010;39:940-2. [ Links ]
5. Oku T, Maeda M, Waga E, Wada Y, Nagamachi Y, Fujita M, et al. Cytomegalovirus cholangitis and pancreatitis in an immunocompetent patient. J Gastroenterol 2005;40:987-92. [ Links ]
6. Velásquez J, Gancedo E, Fainboim H, Kuo L, Marta E, Etchart C, et al. Strategies for the treatment of AIDS-associated sclerosing cholangitis. Am J Med 2004;116:569-70. [ Links ]
7. Kim YS, Cho YD, Lee JS, Jin SY, Shim CS. Cytomegalovirus infection in an HIV patient with duodenal papillitis. Endoscopy 2007;39(Supl. 1):E23. [ Links ]
8. Liong SY, Sukumar SA. Case of the month: An African woman presenting with acalculous cholecystitis and sclerosing cholangiopathy. Br J Radiol 2009;82:699-703. [ Links ]
9. Chen XM, Keithly JS, Paya CV, LaRusso NF. Cryptosporidiosis. N Engl J Med 2002;346:1723-31. [ Links ]
10. Springer KL, Weinberg A. Cytomegalovirus infection in the era of HAART: Fewer reactivations and more immunity. J Antimicrob Chemother 2004;54:582-6. [ Links ]
11. Varani S, Muratori L, De Ruvo N, Vivarelli M, Lazzarotto T, Gabrielli L, et al. Autoantibody appearance in cytomegalovirus-infected liver transplant recipients: Correlation with antigenemia. J Med Virol 2002;66:56-62. [ Links ]
12. Andersen P, Andersen HK. Smooth-muscle antibodies and other tissue antibodies in cytomegalovirus infection. Clin Exp Immunol1975;22:22-9. [ Links ]
13. Waters A, Langlois V, Thorner P, Geary D. Atypical p-ANCA is not a poor prognostic marker in Postinfectious Glomerulonephritis. Pediatr Nephrol 2007;22:1383-6. [ Links ]
14. Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet 2006;368:404-18. [ Links ]
15. Castiella A, Iribarren JA, López P, Arrizabalaga J, Rodríguez F, von Wichmann MA, et al. Ursodeoxycholic acid in the treatment of AIDS-associated cholangiopathy. Am J Med 1997;1032:170-1. [ Links ]
![]() Correspondence:
Correspondence:
Gonzalo Blasco Morente.
Infectious Diseases Unit.
Hospital Universitario Virgen de las Nieves.
Avda. de las Fuerzas Armadas, 2.
18014 Granada, Spain
e-mail: gonzaloblascomorente@gmail.com
Received: 24-01-2013
Accepted: 07-05-2013











 texto en
texto en