My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.106 n.6 Madrid Jun. 2014
CLINICAL NOTE
Phlegmonous gastritis: A rare entity as a differential diagnostic of an acute abdomen. Description of a case and a bibliographic review
Gastritis flemonosa: una entidad poco frecuente como diagnóstico diferencial del abdomen agudo. Descripción de un caso y revisión bibliográfica
Arantzazu Rada-Palomino1, Arantxa Muñoz-Duyos1, Noelia Pérez-Romero1, Harold Vargas-Pierola1, Noelia Puértolas-Rico1, Laura Ruiz-Campos2, Jorge Espinós-Pérez1 and Enrique Veloso-Veloso1
Departments of 1General Surgery and 2Digestive. Hospital Universitario Mútua Terrassa. Barcelona, Spain
ABSTRACT
Phlegmonous gastritis is a rare bacterial infection of the gastric wall, which progress rapidly. It is characterized by a purulent inflammation that can affect the entire gastrointestinal tract and presents a high mortality rate. We are reporting a case of phlegmonous gastritis in an HIV-seropositive man successfully treated with antibiotics. Moreover, a review of the English and Spanish literature is carried out, from 1980 to the present time. The most frequently involved microorganism is Streptococcus spp. (57 %), but the polimicrobial infection is also frequent (17 %). The most important symptom is the intensive epigastric pain associated with vomits and most cases were diagnosed by CT and/or fibrogastroscopy. There are many existing risk factors described. The main one is the immunesuppression, although in 40 % of the cases no risk factors were identified. The global mortality is 27 % without identifying significant differences between antibiotics and surgical treatment, for that reason it is recommended to initiate antibiotic treatment right from the beginning and postponing surgery for the refractory cases and complications.
Key words: Phlegmonous gastritis. Streptococcus A infection.
RESUMEN
La gastritis flemonosa es una infección bacteriana poco frecuente y rápidamente progresiva de la pared gástrica. Se caracteriza por una inflamación purulenta que puede afectar a todo el tracto gastrointestinal y que presenta un índice elevado de mortalidad. En este trabajo se comunica un caso de gastritis flemonosa en un paciente seropositivo para la infección por VIH tratado exitosamente con antibioticoterapia. Además, se realiza una revisión de los casos publicados en la bibliografía médica, en inglés y español desde 1980 hasta la actualidad. El microorganismo más frecuentemente implicado es Streptococcus spp. (57 %), pero también destaca la infección polimicrobiana (17 %). El síntoma más común es el dolor epigástrico intenso asociado a vómitos y la mayoría de casos fueron diagnosticados mediante TC y/o endoscopia. Existen numerosos factores de riesgo descritos, el principal es la inmunosupresión, aunque en el 40 % de los casos no se identificó ningún factor de riesgo. La mortalidad global es del 27 %, sin identificar diferencias significativas entre el tratamiento antibiótico y quirúrgico, por lo que se recomienda instaurar el tratamiento antibiótico de manera precoz y reservar la cirugía para los casos refractarios y las complicaciones.
Palabras clave: Gastritis flemonosa. Infección por Streptococcus A.
Introduction
Phlegmonous gastritis is a rare entity which has a high mortality rate in spite of being treated from an early stage. It consists of a bacterial infection of the gastric wall, local or disseminated, which can produce a purulent discharge. It is caused mainly by Streptococcus spp., although many other microorganisms have been found. The main risk factor is the immunosuppression or the record of any invasive procedure, but many patients do not present known risk factors (1-3). We are reporting an isolated case that occurred in our hospital and a review of the English and Spanish literature is carried out from 1980.
Case report
A 62-year-old male consulted our Emergency Department because of a sudden intense epigastric pain, with a three hours evolution and that had initiated 3 days before and was associated with aqueous diarrhea, vomits and an episode of hematemesis. As an isolated clinical antecedent the patient was HIV-seropositive, he was following an antiretroviral therapy and the last measurement of CD4 was 340-450 cell/mL with an undetectable viral charge.
When the patient arrived at the emergency department, he presented a general deterioration, pallor mucocutaneous and profuse sweating. His vital functions were stable (blood pressure 100/60 mmHg, heart rate 85 bpm) and his body temperature was 37.4 oC. His abdomen was soft but painful under epigastric pressure and showed signs of peritoneal irritation at this level. The blood test showed acute renal failure (creatinine 4.1 mg/dL), leukocytosis with neutrophilic left shift (leukocytes, 6.20 x 109/L, 71 % neutrophils and 18 % bands), C-reative protein, 362.4 mg/L, procalcitonin, 33 mg/mL and metabolic acidosis (pH, 7.20; pCO2: 41 mm Hg; HCO3, 16 mmol/L; EB -12 mmol/L). The ECG and the abdominal and thoracic X-rays did not show any alterations. During his stay in the emergency department, the patient evolved quickly to a hemodynamic instability, developing a septic shock, needing to begin resuscitation measures and empirical antibiotic treatment with cefotaxime and metronidazole.
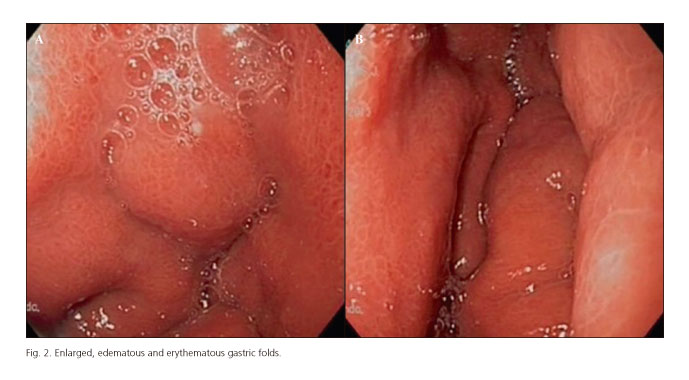
An abdominal CT was performed that showed a diffuse thickening of the gastric wall and the antral region with changes in the density of the fat covering the greater curvature, little free liquid and absence of pneumoperitoneum (Fig. 1). With these findings, a fibrogastroscopy was carried out, which showed increased, edematous and erythematous gastric folds, findings compatible with phlegmonous gastritis (Fig. 2), furthermore an esophagitis with Los Angeles grade C.
Due to the initial hemodynamic instability and the acute renal failure with oliguria, the patient was admitted into the intensive care unit, needing support with vasoactive drugs during the first 24 hours, invasive mechanical ventilation, hemodiafiltration and parenteral nutrition.
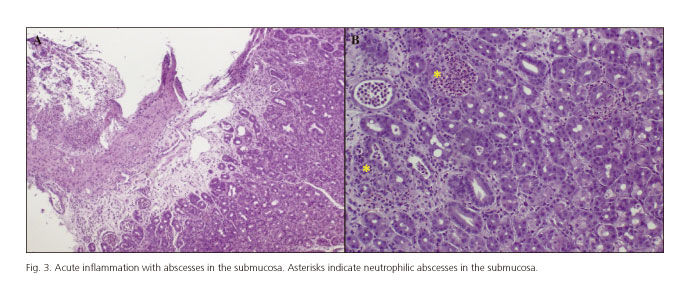
The histological study of the gastric biopsies showed an acute inflammation with abscesses in the submucosa, which confirmed the diagnosis of the phlegmonous gastritis (Fig. 3). The culture of the biopsies and the hemoculture were positive for Streptococcus pyogenes. Therefore, the antibiotic treatment was modified to penicillin G and clindamycin according to antibiogram.
As a secondary complication to the resuscitation measures, the patient developed an acute pulmonary edema and pneumonia. The subsequent evolution was favorable and the patient was discharged after 28 days in hospital with no new abdominal symptoms.
Discussion
Phlegmonous gastritis is a rare entity mentioned for the first time in 1862 by Cruveilhier (2) and, since then, about 500 cases have been published. We have carried out a bibliographic search using the data base Pubmed, with the key words "Phlegmonous gastritis" and "Supurative gastritis". From 1980 to 2013, 45 cases have been reported in the English and Spanish literature, which are summarized in Table I. A bivariant analysis was performed comparing the mortality rate according to the presence of the risk factors and the treatment applied. For the bivariant analysis, the statistical program Stat View 4.0. was used, using the exact Fisher test to the qualitative variants, considering a value of p < 0.05 as significant.
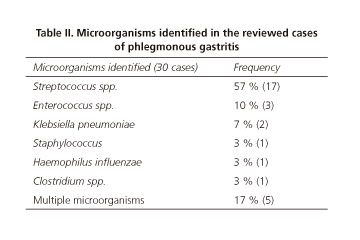
This pathology consists of a purulent inflammation of the gastric wall caused by a bacterial infection. The main pathogen is Streptococcus spp. (especially S. pyogenes) isolated in about 70 % of cases. However, a lot of other microorganisms have been identified as etiologic agents: Staphylococcus spp., Escherichia coli, Haemophilus influenzae, Proteus and Clostridium spp. (3-6). In this review, the pathogen was identified in 67 % of the cases (30/45). Streptococcus spp. was isolated in 57 %. We can also highlight the percentage of cases of polymicrobial infection, 17 % (Table II).
The symptoms of phlegmonous gastritis usually set in 24 hours, although they can develop during several days. They include abdominal pain, which can be very intense and usually located in the epigastric region, nauseas, vomits, fever with or without feverish chill, diarrheas and hematemesis (4-7). All these are very unspecific signs and symptoms, and for this reason a high index of suspicion is required for the diagnosis of this entity, which must be considered in the differential diagnostic of the acute abdomen. Some authors describe the purulent vomits as pathognomonic, although these did not appear in any of the 45 reviewed cases.
About half of the patients affected by phlegmonous gastritis have immunosuppressor factors such as alcoholism, diabetes mellitus, HIV, chronic hepatitis B or treatment with corticoids. The phlegmonous gastritis has also been described as a very rare complication after gastric biopsies or any other invasive procedure. It is believed that these factors predispose the gastric infection eliminating several mechanisms of defense. However, the exact pathogenesis of phlegmonous gastritis is unknown and predisposing factors are identified in 60% of the reviewed cases, being the mortality rate of this group of 33 %. No risk factors were identified in 40 % of the cases and the mortality rate of this group was 17 % (NS). Among the risk factors identified in the review are included alcoholism, HIV, BHV, diabetes mellitus, neoplasms, immunosuppressive treatment, gastric biopsies, esophagectomy and other invasive procedures, gastric or in other locations, performed in the previous weeks to the initiation of the symptoms.
The histology of this entity shows an acute inflammation of gastric submucosa. Furthermore, the culture of the biopsies permits the isolation of the responsible pathogen and all this makes the differential diagnostic with other entities such as the gastric carcinoma, the MALT lymphoma, the GIST, the leiomyoma or the carcinoid tumor (5). However, imaging tests such as abdominal ultrasonography or CT, which reveal thickening of the gastric wall with involvement of the adjacent fat (11,13), as well as the fibrogastroscope, which shows the presence of edematous and erythematosus gastric folds with fibrinopurulent discharge. These finding offer us a highly suspicious diagnosis allowing us to initiate the treatment as soon as possible, with the aim of improving the possibilities of survival of the patients.
In the first reported cases, the diagnosis was mostly surgical and, consequently, the treatment was mostly surgical drainage with or without gastric resection. From the 80s onwards, coinciding with the development of the CT, the phlegmonous gastritis was being diagnosed sooner and parenteral antibiotics was becoming more common as the single treatment. Out of 45 reviewed cases, 47 % were treated only with parenteral antibiotics and 40 % needed surgical treatment with or without gastric resection; the remaining 13 % were diagnosed during the autopsy and for this reason we have no information about the treatment the received.
In 1919, Sundberg reported a series of 215 cases and the mortality rate was 92 % (14). In posterior publications, the mortality rate has been reducing gradually with the development of antibiotics and the earlier diagnosis (3,14,15). The mortality rate of the 45 reviewed cases of our report was 27 % (12/45). In the group treated with antibiotics, 80% was discharged from the hospital without any abdominal symptoms and the mortality rate was 19 % and, in the group treated with surgery, 6 % present gastrointestinal after-effects and the mortality rate was 11 %. The bivariant analysis did not identify significant differences in the morbi-mortality between surgical and antibiotic treatment, for which reason it is recommended to initiate antibiotic treatment right from the beginning and postpone surgery from the refractory cases and the complications and so avoid gastric resections.
In conclusion, phlegmonous gastritis is a rare entity which has to be considered in the differential diagnosis of the acute abdomen, especially in patients with any predisposing factor and who require a high index of suspicion. The ultrasonography, the abdominal CT and the endoscope are very useful for the initial diagnosis, although the definitive diagnosis will be given by the histology and the culture of the gastric biopsies. The most frequent isolated microorganism is Streptococcus spp. but polymicrobial infection is also frequent. In spite of the reduced mortality rate, this is still 27 %, for which reason it is fundamental to initiate antibiotic treatment as soon as possible and consider surgery in refractory cases and in the presence of complications.
References
1. Schultz MJ, Van der Hulst RW, Tytgat GN. Acute phlegmonous gastritis. Gastrointestinal Endoscopy 1996;44:80-3. [ Links ]
2. O'Toole PA, Morris JA. Acute phlegmonous gastritis. Postgraduate Medical Journal 1988;64:315-6. [ Links ]
3. Kim GY, Ward J, Henessey B, Peji J, Godell C Desta H, et al. Phlegmonous gastritis: case report and review. Gastrointestinal Endoscopy 2005;61:168-74. [ Links ]
4. Tierney LM, Gooding G, Bottles K, Montgomery CK, Fitzgerald FT. Phlegmonous gastritis and Haemophilus influenzae peritonitis in a patient with alcoholic liver disease. Digestive Diseases and Sciences 1987;32:97-101. [ Links ]
5. Guisado Vasco P, Román Pascual A, Perales Rodríguez J, Fernández Delgado E, Hernández Ranz F, Pascual Martín A. Manejo conservador de una patología antigua: gastritis flemonosa aguda. Revista Clínica Española 2010;210:41-3. [ Links ]
6. Kim HS, Hwang JH, Hong SS, Chang WH, Kim HJ, Chang YW, et al. Acute Difuse Phelgmonous Esophagogastritis: A case Report. Journal of Korean Medical Science 2010;25:1532-5. [ Links ]
7. Miller AI, Smith B, Roger AI. Phlegmonous gastritis. Gastroenterology 1975;68:231-8. [ Links ]
8. Ross DA, Vincenti AC. Acute phlegmonous gastritis: a rare condition with potentially common cause. British Journal of Hospital Medicine 1994;52:115-6. [ Links ]
9. Lifton LJ, Schlossber D. Phlegmonous gastritis after endoscopic polypectomy. Annals of Internal Medicine 1982;97:373-5. [ Links ]
10. Zazzo JF, Troche G, Millat B, Aubert A, Bedossa P, Keros L. Phlegmonous gastritis associated with HIV-1 seroconversion. Digestive Diseases and Science 1992;37:1454-9. [ Links ]
11. Staroverov VV, Kisel AT, Sumarokov UA, Kachanova TN. A case of phlegmonous gastritis diagnosed by echography. European Journal of Ultrasound 2001;13:197-200. [ Links ]
12. Sood BP, Kalra N, Suri S. CT features of acute phlegmonous gastritis. Clinical Imaging 2000;24:287-8. [ Links ]
13. Avilés JF, Fernández-Seara J, Bárcena R, Domínguez F, Fernández C, Ledo L. Localized phlegmonous gastritis: endoscopic view. Endoscopy 1988;20:38-9. [ Links ]
14. Sundberg H. Phlegmonous gastritis. The Journal of the American Medical Association 1919;73:802. [ Links ]
15. Starr A, Wilson JM. Phlegmonous gastritis. Annals of Surgery 1957;145:88-93. [ Links ]
16. Nicholson BW, Maull KI, Scher LA. Phlegmonous gastritis: clinical presentation and surgical management. Southern Medical journal 1980;73:875-7. [ Links ]
17. Chen ST, Kawai S, Matsumoto H, Kasahara Y, Umemurra H, Shiraha S, et al. Acute diffuse phlegmonous gastritis. The Japanese Journal of Surgery 1980;10:155-8. [ Links ]
18. Cowan SS, Sablin JG, Mori K. Phlegmonous gastritis: report of a case. The Mount Sinai Journal of Medicine 1983;50:417-9. [ Links ]
19. Hornig D, Kühn H, Stadelmann O, Bötticher R. Phlegmonous gastritis after Indian ink marking. Endoscopy 1983;15:266-9. [ Links ]
20. Blei ED, Abrahams C. Diffuse phelgmonous gastroenterocolitis in a patient with an infected peritoneo-jugular venous shunt. Gastroenterology 1983;84:636-9. [ Links ]
21. Mittleman Re, Suárez RV. Phlegmonous gastritis associated with the acquired immunodeficiency syndrome/pre-acquired immunodeficiency syndrome. Archives of pathology & Laboratory medicine 1985;109:765-7. [ Links ]
22. Bracco E, Sategna-Guidetti C, Durando R, Palestini N. Phlegmonous gastritis associated with a very high serum CPK level. Journal of Clinical Gastroenterology 1987;9:364-5. [ Links ]
23. Cruz FO, Soffia PS, Del Río PM, fava MP, Duarte IG. Acute phlegmonous gastritis with mural abscess: CT diagnosis. American Journal of Roentgenology 1992;159:767-8. [ Links ]
24. Van Leeuwen ML, Tjiong HL, Van Blankenstein M, Mulder AH, Bakkeer CM. Phlegmonous gastritis: A unusual presenting symptom of Sjögren's syndrome. Gut 1993;34:1142-4. [ Links ]
25. Wakayama T, Watanabe H, Ishizaki Y, Okuyama T, Ogata H, Tanigawaa K, et al. A case of phlegmonous eshophagitis associated with a diffuse phlegmonous gastritis. The American Journal of Gastroenterology 1994;89:804-6. [ Links ]
26. Hsu CY, Liu JS, Chen DF, Shih CC. Acute diffuse phlegmonous esophagogastritis: report of a survived case. Hepatogastroenterology 1996;43:1347-52. [ Links ]
27. Radhi J, Kamouna M, Nyssen J. Phlegmonous gastritis following coronary bypass surgery. Canadian Journal of Gastroenterology 1999;13:837-9. [ Links ]
28. Jaballah S, Sabri Y, Yacooubi Mt, Mustapha K. Phlegmonous gastritis complicated by upper digestive hemorrhage. Digestive Diseases and Sciences 1999;44:2435-8. [ Links ]
29. Iwakiri Y, Kabemura T, Yasuda D, Okabe H, Soejima A, Soejiima A, et al. A case of acute phlegmonous gastritis successfully treated with antibiotics. Journal of Clinical Gastroenterology 1999;28:175-7. [ Links ]
30. Joko T, TanaKa H, Hirakata H, Henzan H, Hizawa K, Hirakata E, et al. Phlegmonous gastritis in a haemodialysis patient with secondary amyloidosis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and transplant Association - European renal Association 1999;14:196-8. [ Links ]
31. Hu DC, McGrath KM, Jowell PS, Killenberg PG. Phlegmonous gastritis: successful treatment with antibiotics and resolution documented by EUS. Gastrointestinal Endoscopy 2000;52:793-5. [ Links ]
32. Cohen ME, Taylor MB. Treatment of phlegmonous gastritis. Journal of Clinical Gastroennterology 2000;30:100. [ Links ]
33. Yu QQ, Tarig A, Unger SW, Cabello-Inchausti B, Robinson MJ. Phlegmonous gastritis associated with Kaposi sarcoma: a case report and review of the literature. Archives of pathology & Laboratory medicine 2004;128:801-3. [ Links ]
34. Lee BS, Kim SM, Seong JK, Kim SH, Jeong HY, Lee HY, et al. Phlegmonous gastritis after endoscopic mucosal resection. Endoscopy 2005;37:490-3. [ Links ]
35. Harikumar R, Pramod K, Pushpa M, Simi K, Arun G. Gastric lymphoma presenting as phlegmonous gastritis. Journal of gastrointestinal Cancer 2007;38:24-7. [ Links ]
36. Hommel S, Savoye G, Lorenceau-Savale C, Cosstaglioli B, Baron F, Le Pessot F, et al. Phlegmonous gastritis in a 32-week pregnant woman managed by conservative surgical treatment and antibiotics. Digestive Diseases and Sciences 2007;52:1042-6. [ Links ]
37. Corti M, Metta H, Palmieri O, Schtirbu R. Acute phlegmonous gastritis in a patient with AIDS. Enfermedades Infecciosas y Microbiología Clínica 2007;25:218-20. [ Links ]
38. Ajibe H, Osawa H, Yoshizama M, Yamamoto H, Satoh K, Koinuma K, et al. Phlegmonous gastritis after endoscopic submucosal dissection for early gastric cancer. Therapeutic advances in gastroenterology 2008;1:91-5. [ Links ]
39. Guo J, Young SK, Lorenzo CR, Lee CM Kanel GC, Brynes RK, et al. Phlegmonoous gastritis in a patient with a myeloid sarcoma: a case report. Applied immunohistochemistry & molecular morphology 2009;17:458-62. [ Links ]
40. Rajendran S, Baban C, Lee G, Murpy M, O'Hanlon D. Rapid resolution of phlegmonous gastritis using antibiotics alone. BMJ case reports 2009;doi:10.1136/bcr.02.2009.1541. [ Links ]
41. Park CW, Kim A, Cha SW, Jung SH, Yang HW, Lee HI, et al. A case of Phlegmonous Gastritis associated with marked distension. Gut and Liver 2010;4:415-8. [ Links ]
42. Paik DC, Larson JD Johnson SA, Sahm K, Sheweiki E, Fulda GJ. Phlegmonous gastritis and group A streptococcal toxic shock syndrome in a patient following functional endoscopic sinus surgery. Surgical infections 2010;11:545-9. [ Links ]
43. Munroe CA, Chen A. Suppurative (phlegmonous) gastritis presenting as a gastric mass. Digestive Diseases and Sciences 2010;55:11-3. [ Links ]
44. Itonaga M, Ueda k, Ichinose M. Phlegmonous gastritis caused by endoscopic ultrasound-guided fine-needle aspiration (EUS- FNA). Digestive endoscopy: Official journal of the Japan Gastroenterological Endoscopy Society 2012;24:488. [ Links ]
45. Fan JQ, Liu DR, Li C, Chen G. Phlegmonous gastritis after esophagectomy: A case report. World Journal of Gastroenterology 2013;19: 1330-2. [ Links ]
46. Liu YJ, Siracuse JJ, Gage T, Hauser CJ. Phlegmonous gastritis presenting as portal venous pneumatosis. Surgical Infections 2013;14:221-4. [ Links ]
![]() Correspondence:
Correspondence:
Arantzazu Rada Palomino.
Department of General Surgery.
Hospital Universitario Mutua Terrassa.
Plaza del Doctor Robert, 5.
08221 Terrasa, Barcelona. Spain
e-mail: arada@mutuaterrassa.es
Received: 22-01-2014
Accepted: 28-02-2014











 text in
text in