Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.106 no.8 Madrid dic. 2014
Three cases of liver toxicity with a dietary supplement intendedto stop hair loss
Tres casos de hepatotoxicidad por un suplemento dietético destinado a frenar la caída del cabello
Julieta Fernández, Carmen Navascués, Gino Albines, Lissa Franco, María Pipa and Manuel Rodríguez
Hepatology Unit. Department of Digestive Diseases. Hospital Universitario Central de Asturias. Oviedo, Spain
ABSTRACT
Liver toxicity associated with herbal remedies and dietary supplements is an increasing concern. Several toxic hepatitis cases have been reported in the literature in association with products intended for weight loss where green tea extracts are an ingredient.
Three hepatotoxicity cases are reported below in association with the use of Inneov masa capilar®, a dietary supplement intended to stop hair loss whose primary component is green tea catechins. In all of them, other potential causes of acute hepatitis were ruled out.
We highlight the importance of awareness regarding these substances at history taking in order to identify and report hepatic adverse reactions secondary to apparently safe herbs as described in the present manuscript.
Key words: Hepatotoxicity. Green tea. Acute hepatitis. Camellia Sinensis.
RESUMEN
La toxicidad hepática asociada al uso de productos de herboristería y suplementos nutricionales es un fenómeno creciente. En la literatura se han comunicado varios casos de hepatitis tóxica en relación con productos utilizados para la pérdida de peso que incluyen extractos de té verde en su composición.
A continuación se describen tres casos de hepatotoxicidad relacionados con la toma de Inneov masa capilar®, un suplemento nutricional destinado a detener la caída del cabello cuyo componente principal son las catequinas de té verde. En todos ellos se descartaron otras posibles causas de hepatitis aguda.
Recalcamos la importancia de incluir la ingesta de este tipo de sustancias a la hora de realizar la anamnesis para poder detectar y notificar las reacciones hepáticas adversas secundarias a productos herbales de apariencia inocua, como el descrito en el manuscrito.
Palabras clave: Hepatotoxicidad. Té verde. Hepatitis aguda. Camellia Sinensis.
Introduction
Drug-induced liver toxicity is a not so uncommon, potentially serious condition that requires a high level of diagnostic suspicion for want of specific clinical, laboratory, and pathology markers (1). Of late, an increase in acute toxic hepatitis cases associated with herbal products and dietary supplements usually intended for weight loss has been reported. The use of these products has increased in the last few years partly due to mistaken notions about their safety, despite their lack of comprehensive safety controls as opposed to provisions for drug registration (2).
Three cases of acute hepatitis are reported below as related to Inneov masa capilar®, a product intended to stop hair loss that contains green tea catechins.
Materials and methods
We report three cases of acute hepatitis of toxic etiology seen at the Hepatology Unit of a third-level hospital between September 2010 and October 2012.
Causality was established based on temporal relationship with treatment onset, outcome following treatment discontinuation, prior liver toxicity data, and exclusion of alternative etiologies. To this end, in all three cases, history data were collected on consumption of alcohol, mushrooms, molluscs, and raw meat or fish; trips abroad; risky sexual behaviors; blood transfusions or recent medical procedures; and use of drugs, herbal remedies or dietary supplements during the last three months. Lab tests included CBC, coagulation, and complete chemistry; immune status including autoantibodies (anti-nuclear, anti-mitochondrial, anti-smooth muscle, anti-LKM, anti-neutrophil cytoplasmic, anti-SLA, and anti-LC1), immunoglobulins (IgG, IgA, IgM), and serology (IgM antibodies against hepatitis A, B, and E viruses, cytomegalovirus, and Epstein-Bar virus; hepatitis B surface antigen, and antibodies against hepatitis C virus). All three patients underwent abdominal ultrasounds, and the first patient also had a US-guided percutaneous liver biopsy performed.
Patients
The clinical and biological characteristics of these three patients are summarized in table I. All three cases were reported to the relevant regulatory agencies, and all three patients gave their consent to the publication of this manuscript. As regards epidemiology, all three reported they were taking Inneov masa capilar® at a dose of 2 daily tablets when manifestations began. They had no other relevant epidemiologic history, and all three had other causes of acute hepatitis ruled out.
Case report 1
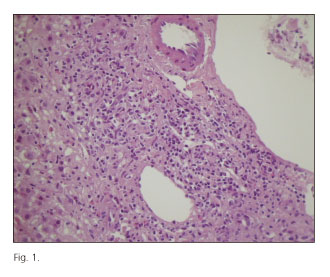
A 59-year-old woman with no relevant medical history presented in September 2010 with malaise followed by jaundice, choluria and fecal acholia for 15 days, which had started during her holidays in Ibiza. Her relevant history only included Inneov masa capilar® for one month before symptom onset. Her physical exam revealed no significant findings but for skin-mucosal jaundice. Lab results are included in table I. Immune markers and viral serology were negative. Abdominal ultrasounds revealed a fatty liver with no other changes. As lab abnormalities persisted and the etiology remained unknown a liver biopsy was decided upon, which revealed portal area enlargement with severe mixed inflammatory infiltration (lymphocytes, polymorphonuclear cells, eosinophils) disrupting the basement membrane, severe ductular proliferation, and lobular damage with multiple necrosis areas, Councilman bodies and cholestasis (Figs. 1 and 2). Although findings were consistent with toxic hepatitis, an empiric therapy was initiated with prednisone 40 mg daily in view of its torpid course, with significant improvement of liver test parameters on the fifth day after treatment onset. Steroid therapy was tapered for 6 months, with normal liver test results after this period of time. They remained normal during follow-up for 14 months.
For this case report causality was probable according to the CIOMS/RUCAM scale.
Case report 2
A 56-year-old woman with diabetes and dyslipidemia presented at the ER in October 2012 complaining of malaise, joint pain, nausea, low back pain, hypogastric pain, and fever. Her epidemiological history included a trip to Sweden within the previous 10 days, and the use of Inneov masa capilar® for the last 23 days. She had also been on ASA and fenofibrate for years now. Her physical examination only revealed epigastric pain. Lab parameters and abdominal ultrasound findings are listed in table I. Viral serology was negative. Autoimmunity was also negative, and immunoglobulins were normal. Her clinical and lab parameters partially improved on the fifth day despite her having not discontinued Inneov masa capilar®. Following its discontinuation liver tests further improved and returned to normal at 3 months after symptom onset.
Case report 3
A 31-year-old woman was referred to us in October 2012 for pruritus, and was admitted to hospital with clinical and laboratory manifestations consistent with non-serious acute hepatitis. History taking revealed a tattoo made 10 years before, and the use of Inneov masa capilar® for the last month. Her physical exam was normal. Lab results and abdominal ultrasounds are summarized in table I. Immunology and viral serology were negative. On the fifth day after Inneov masa capilar® discontinuation manifestations improved, with no pruritus and liver function tests normalization after three months.
Causality for case reports 2 and 3 was highly probable according to the CIOMS/RUCAM scale.
Discussion
Herbal remedies and dietary supplements are responsible for about 10 % of liver toxicity cases reported in the USA (3). This proportion is much higher in Asian countries (4), given their greater use of these products. Two percent of toxic hepatitides recorded in the Registro Español de Toxicidad Hepática are related to herbal remedies; of these, Camellia Sinensis, the plant from which tea is obtained, is the primary causal agent involved (23 % of cases) (5).
Inneov masa capilar® is a product intended to treat hair loss that contains green tea catechins (27-30 %), grape seed catechins (11 %), taurine (11 %), and zinc gluconate. Of these, green tea catechins alone have been involved in hepatotoxicity reactions. The most abundant active ingredients in green tea include polyphenols, mainly flavones (including catechins) and flavonoids, and phenolic acids. The pathogenesis of green tea-related hepatotoxicity is unknown, though likely associated with a metabolic idiosyncrasy mechanism (6).
Many cases of liver toxicity are reported in the literature in connection with the use of extracts from Camellia Sinensis. Exolise®, one such hydroalcoholic extract, was marketed for the treatment of obesity and then withdrawn in 2003 following the report of multiple acute hepatitis cases in France and Spain (7). Over 30 additional cases of liver toxicity have been subsequently reported in association with a number of products containing green tea extracts (8).
As with most cases reported, two of our patients had a predominantly hepatocellular injury pattern (cases 1 and 3: with an ALT/AP ratio of 23.91 and 2.45, respectively). However, case 2 had a predominantly cholestatic pattern with an ALT/AP ratio < 2 (0.46).
In the literature a patient was reported with fulminant hepatitis requiring liver transplantation, but most individuals have a benign course with liver parameters that return to normal after product discontinuation, as was the case with the three patients reported herein (8,9).
While 30 % of the reported cases have hypersensitivity data, only case 2 among our patients had fever, and case 3 presented with itching in the absence of skin lesions. None of the three cases had eosinophilia. It must also be highlighted that all three cases involve women, as occurs with literature reports, probably since women are more inclined to using herbal remedies and dietary supplements.
Reaching a definitive diagnosis of toxic hepatitis remains a challenge for clinicians as this is a diagnosis of exclusion despite the various methods designed in the last few decades based on probabilistic approaches or algorithmic scales, both general and specific, to reach this end (10,11).
In the above case reports diagnosis was solely based on clinical criteria. However, when using the CIOMS/RUCAM diagnostic scale, causality is probable for case 1 and highly probable for cases 2 and 3. All three share a temporal clinical pattern with manifestations arising after one month of use, and liver tests improved for all of them following product discontinuation. In case 1, where the course was torpid, steroid therapy was initiated empirically and probably contributed to an improved outcome. Toxic and autoimmune hepatitides have common clinical, laboratory, and serologic parameters that make differentiation difficult, to the extent that in some patients a definitive diagnosis may only be reached by observing the outcome after steroid discontinuation (12). The fact that our patient had no recurrent transaminase increase on steroid discontinuation supports a toxic rather than autoimmune origin (12). Also relevant is the initial chemistry improvement seen in case 2, before Inneov® withdrawal. However, this fact does not rule out a potential product contribution to disease onset (13). Furthermore, other causes of acute hepatitis were comprehensively excluded in all three patients, including hepatitis E virus, which has been of late recognized as the cause of some hepatitis cases wrongly labeled as toxic (14).
Hepatitis cases thus far reported in connection with green tea extracts mainly derive from their use regarding weight loss. Only one case had been reported of a 41-year-old woman who had acute hepatitis after taking Densitive®, a natural product containing Camellia Sinensis (27 %) that, as Inneov masa capilar®, is indicated to stop hair thinning (15).
Liver toxicity from herbal and dietary supplements is not uncommon, and probably remains underdiagnosed. Prevention requires a multidimensional strategy including more safety information to consumers and health providers alike, improved adverse event reporting, and increased regulations regarding their production, marketing, and consumption.
References
1. Meier Y, Cavallaro M, Roos M, Pauli-Magnus C, Folkers G, Meier PJ, et al. Incidence of drug-induced liver injury in medical inpatients. Eur J Clin Pharmacol 2005;61:135-43. [ Links ]
2. Prieto J, Gómez J, Franco S. Hepatitis tóxica por Camellia Sinensis. Gastroenterol Hepatol 2008;31:402-4. [ Links ]
3. Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008;135:1924-34. [ Links ]
4. Zhou Y, Yang L, Liao Z, He X, Zhou Y, Guo H. Epidemiology of drug-induced liver injury in China: a systematic analysis of the Chinese literature including 21.789 patients. Eur J Gastroenterol Hepatol 2013;25:825-9. [ Links ]
5. García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, et al. Liver injury induced by "natural remedies": An analysis of cases submitted to the Spanish Liver Toxicity Registry. Rev Esp Enferm Dig 2008;11:688-95. [ Links ]
6. Herrera S, Bruguera M. Hepatotoxicidad inducida por el uso de hierbas y medicamentos para perder peso. Gastroenterol Hepatol 2008;31:447-53. [ Links ]
7. Vial T, Bernard G, Lewden B, Dumortier J, Descotes J. Acute hepatitis due to Exolise, a Camellia sinensis derived drug. Gastroenterol Clin Biol 2003;27:1166-7. [ Links ]
8. Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur J Clin Pharmacol 2009;65:331-41. [ Links ]
9. Molinari M, Watt K D S, Kruszyna T, Nelson R, Walsh M, Huang W-Y, et al. Acute liver failure induced by green tea extracts: Case report and review of the literature. Liver Transpl 2006;12:1892-5. [ Links ]
10. Tesche R, Eickhoff A, Wolff A, Frenzel C, Schulze J. Herbal hepatotoxicity and WHO global introspection method. Ann Hepatol 2013;12:11-21. [ Links ]
11. García-Cortés M, Stephens C, Lucena MI, Fernández-Castañer A, Andrade RJ. Causality assessment methods in drug induced liver injury: Strengths and weaknesses. J Hepatol 2011;55:683-91. [ Links ]
12. Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci 2011;56:958-976. [ Links ]
13. Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol 2008;14:6774-85. [ Links ]
14. Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology 2011;141:1665-72. [ Links ]
15. Velhelst X, Burvenich D, Van Sassenbroeck D, Gabriel C, Lootens M, Baert D. Acute hepatitis after treatment for hair loss with oral green tea extracts (Camellia Sinensis). Acta Gastroenterol Belg 2009;72:262-4. [ Links ]
![]() Correspondence:
Correspondence:
Julieta Romina Fernández Molina
Hepatology Unit. Department of Digestive Diseases
Hospital Universitario Central de Asturias
C/ Celestino Villamil, s/n
33006. Oviedo, Spain
e-mail: jrfernandezmolina@hotmail.com
Received: 07-11-2014
Accepted: 23-06-2014











 texto en
texto en