Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.1 Madrid ene. 2015
Proton pump inhibitor-responsive esophageal eosinophilia: A historical perspective on a novel and evolving entity
Esofagitis eosinofílica respondedora a los inhibidores de la bomba de protones: perspectiva histórica sobre una entidad nueva y en desarrollo
Javier Molina-Infante1, David A. Katzka2 and Evan S. Dellon3
1Department of Gastroenterology. Hospital San Pedro de Alcántara. Cáceres, Spain.
2Division of Gastroenterology and Hepatology. Mayo Clinic. Rochester, Minnesota. USA.
3Center for Esophageal Diseases and Swallowing. University of North Carolina School of Medicine. Chapel Hill, North Carolina. USA
ABSTRACT
Eosinophilic esophagitis (EoE) is an emerging chronic esophageal disease, first described in 1993, with a steadily increasing incidence and prevalence in western countries. Over the 80's and early 90's, dense esophageal eosinophilia was mostly associated gastroesophageal reflux disease (GERD). For the next 15 years, EoE and GERD were rigidly considered separate entities: Esophageal eosinophilia with pathological acid exposure on pH monitoring or response to proton pump inhibitor (PPI) therapy was GERD, whereas normal pH monitoring or absence of response to PPIs was EoE. Updated guidelines in 2011 described a novel phenotype, proton pump inhibitor-responsive esophageal eosinophilia (PPI-REE), referring to patients who appear to have EoE clinically, but who achieve complete remission after PPI therapy. Currently, PPI-REE must be formally excluded before diagnosing EoE, since 30-40 % of patients with suspected EoE are eventually diagnosed with PPI-REE. Interestingly, PPI-REE and EoE remain undistinguishable based on clinical, endoscopic, and histological findings, pH monitoring, and measurement of tissue markers and cytokines related to eosinophilic inflammation.
This review article aims to revisit the relatively novel concept of PPI-REE from a historical perspective, given the strong belief that only GERD, as an acid peptic disorder, could respond to the acid suppressing ability of PPI therapy, is becoming outdated. Evolving evidence suggests that PPI-REE is genetically and phenotypically undistinguishable from EoE and PPI therapy alone can almost completely reverse allergic inflammation. As such, PPI-REE might constitute a subphenotype of EoE and PPI therapy may be the first therapeutic step and diet/ steroids may represent step up therapy. Possibly, the term PPI-REE will be soon replaced by PPI-responsive EoE. The mechanism as to why some patients respond to PPI therapy (PPI-REE) while others do not (EoE), remains to be elucidated.
Key words: Proton pump inhibitor-responsive esophageal eosinophilia. Eosinophilic esophagitis. Gastroesophageal reflux disease. Proton pump inhibitor.
ABBREVIATIONS
EoE: Eosinophilic esophagitis.
GERD: Gastroesophageal reflux disease.
IL: Interleukin.
PPI: Proton pump inhibitor.
PPI-REE: Proton pump inhibitor-responsive esophageal eosinophilia.
STAT6: Signal transducer and activator of the transcription 6.
Introduction
Eosinophilic esophagitis (EoE) is a chronic, immune/antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation (1). Since the first descriptions in the early 1990s (2,3), it has become an emerging cause of esophageal symptoms worldwide. Currently, it represents the second most common cause of esophageal inflammation after gastro-esophageal reflux disease (GERD) and the leading cause of dysphagia and food impaction in children and young adults (4). Furthermore, its incidence has steadily risen (4) and prevalence rates have been consistently reported in Europe and the USA in the 44 to 56 cases per 100,000 inhabitants range (5,6), comparable to that of pediatric Crohn's disease in western countries.
Rapid advances in the diagnosis and management of EoE have been accomplished over the last seven-year period, as reflected by updated consensus guidelines in 2007, 2011 and 2013 (1,7,8). One of the major breakthroughs has been the description of a new potential disease phenotype, proton pump inhibitor-responsive esophageal eosinophilia (PPI-REE) (1). This novel phenotype refers to patients with symptoms and histological findings suggestive of EoE who achieve complete remission on PPI therapy. The categorization of PPI-REE has led to significant changes in the way we usually view and study esophageal eosinophilia, as it differs from earlier data suggesting that the presence of intraepithelial esophageal eosinophils was pathognomonic for GERD (9-12).Views of PPI-REE continue to evolve from placing a rigid distinction between GERD and EoE in 2007 (7) to more recent evidence suggesting that PPI-REE and EoE may be closely related and that GERD may be a co-factor for EoE in some patients.This review aims to describe the history of PPI-REE and to summarize potential pathogenetic pathways proposed to explain responsiveness to PPI therapy.
Historical overview of PPI-REE
The rapidly evolving diagnostic approach to esophageal eosinophilia over the last three decades is summarized in figure 1.
1977-1992: Esophageal eosinophilia is almost pathognomonic for GERD
In 1977 and 1978, the first reports of eosinophilic inflammation of the esophageal epithelium in two adults with dysphagia and no GERD symptoms were published (9,10). Over the following few years, isolated case reports described additional similar findings in adults and children (11-13). All of these case reports were attributed to a variant of eosinophilic gastroenteritis.During the 1980's and early 90's, however, a number of reports associated intraepithelial eosinophils in esophageal biopsy specimens with GERD (14-17). Therefore, the significance of esophageal eosinophilia was underappreciated since most pathologists viewed the presence of intraepithelial esophageal eosinophils as pathognomonic for GERD. In addition, obtaining esophageal biopsies was not a standard part of adult gastroenterology practice.
In 1993, the first series on EoE as a distinct entity, made up of 11 adults with dysphagia, normal acid exposure on pH monitoring and dense esophageal eosinophilia (> 20 eos/HPF) was reported (2). Seven patients had food hypersensitivity, and all required advanced intervention (dilation and/or steroids in one case) for resolution of symptoms. The authors cautioned about automatically attributing esophageal eosinophilia to GERD.
1993-2007: Esophageal eosinophilia responsive to PPI therapy is GERD
Despite several seminal reports on EoE as a novel entity in 1993 and 1994 (2,3), as well as the first description of the efficacy of dietary modifications (elemental diet) to induce remission of EoE in 1995 (18), EoE was initially viewed as a rare disease and it did not gain recognition in the scientific community for a decade. Starting around 2003, as studies on EoE began to populate the literature, the first consensus guidelines report on eosinophilic esophagitis (EoE) was published in 2007 by the First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees (7). Diagnostic criteria proposed for EoE included: a) Symptoms of esophageal dysfunction, mainly dysphagia/food impaction; b) esophageal eosinophilic infiltration (> 15 eos/HPF); and c) either absence of response to proton pump inhibitor (PPI) therapy or demonstration of normal esophageal acid exposure on pH monitoring. Therefore, it was suggested that either a response to PPI therapy or increased acid exposure on pH monitoring were consistent with gastroesophageal reflux disease (GERD). The premise underlying this recommendation was that GERD, as an acid peptic disorder, responded to the acid suppressing ability of PPI treatment. An illustrative example is provided in 2006 with the first case reports of what would now is recognized as PPI-REE, in which two children and an adult with clinical, endoscopic and histological data suggestive of EoE demonstrated complete resolution of esophageal eosinophilia in response to PPI therapy (19). Interestingly, the authors concluded that "while these patients' presentation was highly suggestive of allergic esophagitis, their symptoms, and the gross and histologic esophageal abnormalities normalized following treatment with a PPI, implicating acid reflux as the underlying cause". By that time, however, some visionary authors raised the possibility that a rigid distinction between GERD and EoE based upon PPI responsiveness might be too simplistic, given the potential mechanisms of interaction between these disorders (20). In fact, these authors recommended a systematic trial of PPI therapy in all patients with an EoE phenotype (as we do today), proposing that a response to PPI therapy would not preclude a diagnosis of EoE. For the period between 2008 and 2010, several small series or case reports in children and adults also highlighted the existence of symptomatic patients who met clinical, endoscopic and histological criteria for EoE with complete clinicopathologic remission on PPI therapy (21-23). Additionally, no differences in the rate of therapeutic response were observed when comparing esomeprazole to topical steroids in adults with a diagnosis of EoE based on the presence of esophageal eosinophilia (24).
2011: The description of PPI-REE as a new phenotype
In 2011, the first prospective series was reported evaluating the efficacy of PPI therapy in 35 patients with upper GI symptoms and esophageal eosinophilia > 15 eos/HPF (25). Twenty-six patients (75 %) had a clinicopathologic remission on treatment with a PPI, including complete remission in 50 % of the patients with a typical EoE phenotype (dysphagia and/or food impaction plus typical endoscopic findings). Patients with EoE and PPI-REE were undistinguishable at baseline by clinical, endoscopic and histologic features. Of note, neither negative nor positive pH monitoring adequately predicted the favorable response to PPI's, questioning the ability of esophageal pH monitoring to discern between GERD and PPI-REE.
The description of this new phenotype, PPI-responsive esophageal eosinophilia (PPI-REE), was acknowledged as one of the major advances in EoE research in the updated 2011 consensus recommendations for EoE (1).
2014: PPI-REE is here to stay, likely as an EoE phenotype
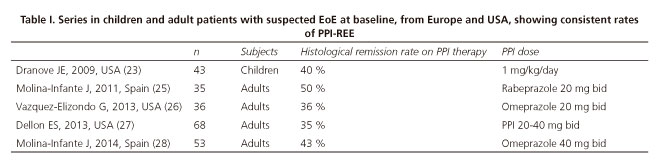
During the past three years, several large series from the USA and Europe have confirmed PPI-REE as a common phenotype among patients with suspected EoE (26-28). The prevalence of PPI-REE in these series ranges from 35 % to 43 %, stressing its importance in clinical practice (Table I). As such, a PPI trial before arriving at a diagnosis of EoE is currently mandatory in order to avoid unnecessary therapeutic interventions in PPI-REE, such as topical steroids or elimination diets.This, as well as the distinction between EoE and esophageal eosinophilia, was highlighted in the recently published third iteration of clinical guidelines (8).
The challenge of distinguishing EoE and PPI-REE
Because a PPI trial followed by a diagnostic endoscopy is required before a diagnosis of EoE is confirmed (1,8), it would be ideal to be able to differentiate EoE from PPI-REE at baseline prior to this PPI trial. Accordingly, there have been a number of efforts aiming to distinguish EoE from PPI-REE.
Clinical, endoscopic and histologic features
At the present time, three studies have failed to find distinguishing baseline clinical, endoscopic and histological features between patients ultimately found to have EoE or PPI-REE after a PPI trial (25,27,29).
Esophageal pH monitoring
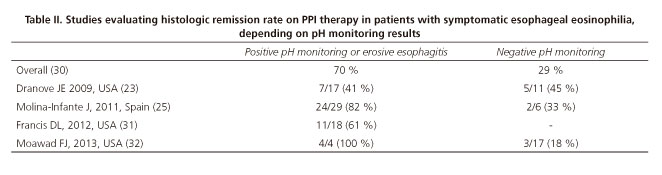
In 2007 EoE guidelines, pH monitoring results were acknowledged as a key diagnostic tools to distinguish EoE from PPI-REE. Nevertheless, we currently know that response to PPI therapy is not accurately predicted by the presence or absence of pathological esophageal acid exposure. It is important to emphasize that PPI responsiveness is significantly higher in patients with documented GERD when compared to those with no GERD on endoscopy/pH monitoring (70 % vs. 29 %, p < 0.001), as shown in the first systematic review on PPI-REE (30). The results of the main studies addressing this issue are shown in table II, highlighting that response to PPI therapy may occur with either normal or pathologic esophageal pH monitoring (30-32).
Tissue markers and cytokines associated to esophageal eosinophilia
More recently, immunohistochemical expression of markers of eosinophilic infiltration and associated inflammatory cells (major basic protein, eotaxin-3 and tryptase) (33) and gene expression of Th2 chemokine (eotaxin-3) and cytokines (interleukin (IL) (IL-5) and IL-13) (28) failed as well to distinguish between PPI-REE and EoE patients.
Gene expression
Identification of candidate genes has been a major step to understand the molecular pathogenesis of EoE (34). TheEoE diagnostic gene panel (EDP) has shown a 96 % sensitivity and 98 % specificity to distinguish patients with EoE in remission from controls, as well as identifying patients exposed to glucocorticoids and likely to have relapse after treatment (35). A recent study has been the first to evaluate differences in EDP between untreated 28 EoE and 36 PPI-REE patients from 5 US centers (36). Of note, the gene upregulation and downregulation pattern comprising the EoE hallmark gene signature was also present in PPI-REE samples at baseline, including increased CCL26 levels for eosinophil chemotaxis, CPA3 levels for mastocytosis, IL-13 responding MUC4 levels, and POSTN levels for tissue remodeling (36). In addition, molecular signature and mastocytosis of untreated PPI-REE were almost reversible by using PPI monotherapy, in concordance with other recent study demonstrating that PPI therapy in PPI-REE downregulated eotaxin-3 and Th2 cytokines gene expression in esophageal tissue, similarly to that seen in steroid-responsive EoE (28).
Advances in understanding PPI-REE pathophysiology
The prevailing hypothesis to explain PPI-REE has been that coexisting GERD might be the priming event, allowing the potential entry of food derived allergenic molecules through acid-induced epithelial barrier damage (20). Thus, GERD-induced epithelial damage could expose the deeper layers of the esophageal squamous epithelium to antigens that ordinarily could not penetrate a normal mucosa. This in turn could lead to facilitated recognition of antigens by antigen presenting cells. However, emerging evidence demonstrates that EoE and PPI-REE groups might be even more closely related asPPI therapy not only restores esophageal epithelial integrity, but may also blunt the inflammatory pathway by downregulation of gene expression of Th2 chemokines and cytokines (28,36). A summary of the interplay between potential pathophysiologic mechanisms and PPI therapeutic effects in PPI-REE is displayed in figure 2.
PPI partially restores epithelial barrier function of the esophageal mucosa
Although the restoration of the esophageal epithelial barrier has been the most accepted hypothesis to explain the existence of PPI-REE, the first study objectively addressing this issue in EoE was published just some months ago (37). Sixteen patients with esophageal eosinophilia > 15 eos/HPF were compared to eleven controls at baseline. Esophageal mucosal integrity was measured in the distal esophagus, in vivo with a through-the-scope electrical tissue impedance spectroscopy probe during endoscopy, and in vitro with 2 biopsies for electron microscopic analysis of dilated intercellular spaces and 4 biopsies for measuring transepithelial electrical resistance and transmucosal flux of fluorescently-labelled molecules sized 0.3 and 40 kDa (similar to size of food allergens) in Ussing chambers. In patients with esophageal eosinophilia, all measurements of mucosal integrity were significantly impaired when compared to controls. Patients with esophageal eosinophilia were then given omeprazole 40 mg twice daily and reevaluated 8 weeks later. After acid-suppressive therapy, mucosal integrity was partially restored in PPI-REE, but not in EoE patients. The authors concluded that mucosal integrity impairment in PPI-REE might be due to GERD, whereas it might be related to inflammatory cell recruitment in EoE. Therefore, this study suggests that exposure of esophageal epithelium to refluxed acid in PPI-REE patients, due to epithelial barrier impairment, may be the first "hit" in the development of esophageal eosinophilia.
Can GERD be due to cytokine-mediated damage from a Th2 immune response?
Reflux esophagitis is believed to be caused by the direct caustic effects of refluxed gastric acid on esophageal epithelial cells. In 2009, a provocative experimental study demonstrated that GERD caused esophageal inflammation through a cytokine-mediated mechanism rather than direct epithelial injury (38). In this study, the authors observed that after surgical induction of reflux, the first histologic inflammatory response detected was a lymphocytic infiltration of the submucosa which progressed to the mucosal surface. Interestingly, mucosal erosions did not appear until postoperative week 4. These findings suggested that reflux esophagitis develops primarily as an immune-related injury rather than solely as a caustic chemical injury. In this regard, a recent experimental study has shown that eotaxin-3 expression in GERD and EoE cell cultures is similar when stimulated with Th2 cytokines (39), raising the possibility that in atopic patients at risk for EoE, injury induced by reflux may be diverted to an alternate inflammatory pathway (Th2 immune response) rather than that typical of erosive esophagitis (Th1 immune response). This hypothesis would explain both the absence of distinguishing features with EoE and response to PPI therapy in PPI-REE.However, more data are needed to fully assess this pathway.
Effects of PPI therapy on esophageal eosinophilia
Eotaxin-3 is a potent eosinophil chemoattractant that plays a key role in trafficking eosinophils to the esophagus in EoE. The expression of eotaxin-3 is stimulated by Th2 cytokines, such as IL-4, IL-5 and IL-13 (normally overproduced in allergic diseases), whose effects are mediated by the signal transducer and activator of the transcription (STAT) 6 signaling pathway (40,41).
Emerging translational research has recently demonstrated that PPIs might blunt eosinophil recruitment, independent of their acid suppressive effect. In 2009, PPIs were shown to inhibit in vitro IL-4 and IL-13 signaling STAT6 in murine asthma, reducing significantly the presence of inflammatory cells in bronchoalveolar lavage fluid and lung sections, including eosinophils (42). In 2013, an experimental study demonstrated that omeprazole blocked Th2 cytokine-stimulated eotaxin-3 expression in esophageal squamous cell cultures from both GERD and EoE patients (39). The same group has subsequently reported that inhibition of IL-4 and IL-13 stimulated eotaxin-3 expression in EoE esophageal cells is mediated through blocking STAT6 (43). As these experiments are conducted using in vitro cultured esophageal epithelial cells, the observed PPI effects were independent of their effects on gastric acid production. Overall, these latter studies suggest PPIs can have anti-inflammatory actions independent of their effects on acid-secretion and cast doubt on the assumption that a positive response to PPI therapy necessarily establishes a diagnosis of GERD. Supporting this line of investigation, a recent study has demonstrated for the first time in vivo that PPI therapy in PPI-REE significantly downregulates gene expression of Th2-inflammatory markers in the distal and proximal esophagus, similarly to findings observed in EoE patients after topical steroids (28). Additionally, a recent study has also shown the ability of PPI therapy to nearly reverse gene expression associated with PPI-REE particularly that associated with classic features of allergic inflammation (36).
Apart from responsiveness to PPI therapy, is there any difference between EoE and PPI-REE?
Evolving evidence, showing EoE and PPI-REE are genetically and phenotypically undistinguishable, now questions whether PPI-REE and EoE are two different diseases, as summarized in a recent editorial (44). It may be counterintuitive to define an EoE subtype by responsiveness to PPI therapy and preferable to define it by clinical and mechanistic characteristics, features that are appearing to be more and more similar to classic EoE. For example, it has been pointed out that we do not define Crohn's disease responsive to azathioprine as a different entity than Crohn's disease that responds to infliximab. Similarly, it is tempting to approach EoE as a disease in which use of PPI's is the first step in treatment and diet and steroids represent step up therapy, especially when no major pathophysiologic differences between PPI-REE and EoE have yet to be found. Therefore, the time to rename PPI-REE as PPI-responsive EoE might be near.
Future perspectives in PPI-REE
A main current issue with PPI-REE is that we do not understand why among patients with identical genotypic and phenotypic presentations, some patients respond to PPI's (PPI-REE) yet others do not (EoE). Following the lead of other diseases, there may be genomic differences or subtle variations in disease pathways that explain therapeutic response and prognosis. In the aforementioned article on genetic differences between EoE and PPI-REE, KCNJ2 (potassium inwardly-rectifying channel, subfamily J, member 2/Kir2.1) became the only gene with significant differential expression between both disorders (36). KCNJ2 resulted in 72 % sensitivity and 72 % specificity to predict PPI-REE-pre versus EoE. KCNJ2 encodes the potassium channel Kir2.1, which is abundant in gastrointestinal mucosa and colocalizes with the H1-K1-ATPase/proton pump. Therefore, the authors propose a potential interaction between this potassium channel and proton pump in the upper gastrointestinal epithelium to explain PPI-REE.
Another important unsolved issue is to ascertain whether PPI-REE and EoE also share a similar natural history and long-term management. EoE has been demonstrated to be a chronic disease with persistence of symptoms and inflammation over years (45). Furthermore, long-standing eosinophilic inflammation may increase the risk of esophageal fibrotic remodeling with subsequent stricture formation. A recent study nicely showed how the prevalence of esophageal strictures correlates with the duration of untreated disease (46). Eosinophils, eotaxin-3 and Th2 cytokines (IL-5 and IL-13) have been shown to be necessary for this esophageal tissue remodeling in EoE. In addition, swallowed topical corticosteroids and dietary interventions not only reduce this inflammation, but may prevent and even reverse this fibrotic remodeling process in the long run (47). In this respect, eosinophil-related inflammation markers in PPI-REE are undistinguishable from EoE at baseline (28,33) and PPI therapy has been shown to significantly downregulate gene expression of Th2 inflammatory mediators in PPI-REE (28). As such, there is concern that PPI-REE and EoE might share a similar risk of fibrostenotic complications if untreated and a hope that PPI therapy might impact this natural history in PPI-REE as well.
The need for long-term efficacy of PPI therapy in PPI-REE patients, the required PPI dose and duration, and the use of CYP2C19 genotype for dosing remain as of yet unknown. Two small retrospectives series of patients have shown that children with PPI-REE may eventually progress to EoE (48,49). A first attempt to answer these questions has been recently presented in abstract form (50). In forty PPI-REE patients, acid suppressive therapy was progressively tapered based on clinical symptoms and maintained at the lowest dose with the target endpoint of clinical remission, with a follow-up endoscopy performed at 12 months or longer. Sustained clinico-histological remission on low-dose maintenance PPI therapy was observed in 64 % of adult PPI-REE patients. A majority of relapsers showed recurrent eosinophilic inflammation limited to the distal esophagus, which resolved after PPI-dose intensification. Therefore, it might be uncommon for adult PPI-REE patients to fully lose PPI response and be reclassified as EoE. Undoubtedly, further studies are required in this field to clarify the long-term management for PPI-REE patients.
Key points
- From a historical point of view, both esophageal eosinophilia and response to PPI therapy have long been considered almost pathognomonic for GERD.
- Over the past seven years, the advent of EoE has dramatically changed our way of diagnosing patients with symptomatic esophageal eosinophilia, albeit PPI response was still considered suggestive of GERD.
- The formal description of PPI-REE in 2011 has made it even more complicated, as neither esophageal eosinophilia nor response to PPI therapy can no longer be considered as pathognomic for GERD.
- At the present time, clinical, endoscopic, histologic, pH monitoring, tissue and genetic markers have failed to distinguish EoE and PPI-REE.
- Therefore, PPI-REE might constitute a subphenotype of EoE with a clinical management more similar to EoE rather than GERD. PPI therapy may be considered the first step in treatment for EoE and diet/steroids may represent step up therapy.
- The mechanisms as to why among patients with an identical baseline genotypic and phenotypic expression, some respond to PPI therapy (PPI-REE) while others do not (EoE), remain yet to be elucidated.
- PPI therapy partially restores impaired esophageal mucosal integrity in PPI-REE, but not in EoE, underscoring the potential role of GERD in PPI-REE patients.
- PPI therapy downregulates both EoE diagnostic panel and eotaxin-3 and Th2 cytokines gene expression in PPI-REE patients, in a similar way to that seen in experimental studies showing anti-inflammatory effects of PPI therapy.
References
1. Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allerg Clin Immunol 2011;128:3-10. [ Links ]
2. Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993;38:109-16. [ Links ]
3. Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vögtlin J. Idiopathic eosinophilic esophagitis: A frequently overlooked disease with typical clinical aspects and discrete endoscopic findings (in German with English abstract). Schweiz Med Wochenschr 1994;33:1419-29. [ Links ]
4. Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M, et al. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol 2011;128:1349-50. [ Links ]
5. Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 2014;12:589-96. [ Links ]
6. Arias A, Lucendo AJ. Prevalence of eosinophilic oesophagitis in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol 2013;25:208-12. [ Links ]
7. Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007;133:1342-63. [ Links ]
8. Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Kaztka DA. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679-92. [ Links ]
9. Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 1977;72:1312-6. [ Links ]
10. Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 1978;74:1298-301. [ Links ]
11. Picus D, Frank PH. Eosinophilic esophagitis. AJR Am J Roentgenol 1981;136:1001-3. [ Links ]
12. Munch R, Kuhlmann U, Makek M, Ammann R, Siegenthaler W. Eosinophilic esophagitis, a rare manifestation of eosinophilic gastroenteritis. Schweiz Med Wochenschr 1982;112:731-4. [ Links ]
13. Matzinger MA, Daneman A. Esophageal involvement in eosinophilic gastroenteritis. Pediatr Radiol 1983;13:35-8. [ Links ]
14. Leape LL, Bhan I, Ramenofsky ML. Esophageal biopsy in the diagnosis of reflux esophagitis. J Pediatr Surg 1981;16:379-84. [ Links ]
15. Winter HS, Madara JL, Stafford RJ, Grand RJ, Quinlan JE, Goldman H. Intraepithelial eosinophils: A new diagnostic criterion for reflux esophagitis. Gastroenterology 1982;83:818-23. [ Links ]
16. Brown LF, Goldman H, Antonioli DA. Intraepithelial eosinophils in endoscopic biopsies of adults with reflux esophagitis. Am J Surg Pathol 1984;8:899-905. [ Links ]
17. Hyams JS, Ricci A Jr, Leichtner AM. Clinical and laboratory correlates of esophagitis in young children. J Pediatr Gastroenterol Nutr 1988;7:52-6. [ Links ]
18. Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: Improvement with an amino acid-based formula. Gastroenterology 1995;109:1503-12. [ Links ]
19. Ngo P, Furuta GT, Antonioli DA, Fox VL. Eosinophils in the esophagus: Peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol 2006;101:1666-70. [ Links ]
20. Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol 2007;102:1301-6. [ Links ]
21. Rodrigo S, Abboud G, Oh D, DeMeester SR, Hagen J, Lipham J, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol 2008;103:435-42. [ Links ]
22. Sayej WN, Patel R, Baker RD, Tron E, Baker SS. Treatment with high-dose proton pump inhibitors helps distinguish eosinophilic esophagitis from noneosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2009;49:1-7. [ Links ]
23. Dranove JE, Horn DS, Davis MA, Kernek KM, Gupta SK. Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J Pediatr 2009;154:96-100. [ Links ]
24. Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci 2010;55:1313-9. [ Links ]
25. Molina-Infante J, Ferrando-Lamana A, Ripoll C, Hernández-Alonso M, Mateos JM, Fernández Bermejo M, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011;9:110-7. [ Links ]
26. Vazquez-Elizondo G, Ngamruengphong S, Khrisna M, Devault KR, Talley NJ, Achem SR. The outcome of patients with oesophageal eosinophilic infiltration after an eight-week trial of a proton pump inhibitor. Aliment Pharmacol Ther 2013;38:1312-9. [ Links ]
27. Dellon ES, Speck O, Woodward K, Covey S, Rusin S, Gebhart JH, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: A prospective cohort study. Am J Gastroenterol 2013 Oct 22. [ Links ]
28. Molina-Infante J, Rivas MD, Hernández-Alonso M, Vinagre-Rodríguez G, Mateos Rodríguez JM, Dueñas-Sadornil C, et al. Remission in proton pump inhibitor-responsive esophageal eosinophilia correlates with downregulation of eotaxin-3 and TH2 cytokines, similarly to eosinophilic esophagitis after steroids. Alimentary Pharmacol Ther 2014; in press. [ Links ]
29. Moawad FJ, Schoepfer AM, Safroneeva E, Ally MR, Chen YJ, Maydonovitch CL, et al. Eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment Pharmacol Ther 2014;39:603-8. [ Links ]
30. Molina Infante J, Katzka DA, Gisbert JP. Review article: Proton pump inhibitor therapy for suspected eosinophilic esophagitis. Aliment Pharmacol Ther 2013;37:1157-64. [ Links ]
31. Francis DL, Foxx-Orenstein A, Arora AS, Smyrck TC, Jensen K, Nord SL, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic esophagitis. Aliment Pharmacol Ther 2012;35:300-7. [ Links ]
32. Moawad FJ, Veerappan GR, Dias JA, Baker TP, Maydonovitch CL, Wong RK. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol 2013;108:366-72. [ Links ]
33. Dellon ES, Speck O, Woodward K, Covey S, Rusin S, Gebhart JH, et al. Markers of eosinophilic inflammation for diagnosis of eosinophilic esophagitis and proton pump inhibitor-responsive esophageal eosinophilia: A prospective study. Clin Gastroenterol Hepatol 2014;12:2015-22. [ Links ]
34. Holvoet S, Blanchard C. Genetic and molecular mechanisms leading to eosinophilic esophagitis. Rev Esp Enferm Dig 2014;106:276-80. [ Links ]
35. Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, Putnam PE, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 2013;145:1289-99. [ Links ]
36. Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol 2014 Oct. 19. doi: 10.1016/j.jaci. 2014.08.043 [ Links ]
37. van Rhijn BD, Weijenborg PW, Verheij J, van der Bergh Weerman MA, Verseijden C, van den Wijngaard, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014;12:1815-23. [ Links ]
38. Souza RF, Huo X, Vittal M, Schuler CM, Carmack SW, Zhang HY, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 2009;137:1776-84. [ Links ]
39. Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut 2013;62:824-32. [ Links ]
40. Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009;137:1238-49. [ Links ]
41. Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, et al. A striking local oesophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol 2011; 127:208-17. [ Links ]
42. Cortes JR, Rivas MD, Molina-Infante J, Gonzalez-Nuiñez MA, Perez-G M, Masa JF, et al. Omeprazole inhibits IL-4 and IL-13 signaling signal transducer and activator of transcription 6 activation and reduces lung inflammation in murine asthma. J Allergy Clin Immunol 2009;124:607-10. [ Links ]
43. Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One 2012;7:e50037. [ Links ]
44. Katzka DA. Eosinophilic esophagitis and proton pump-responsive esophageal eosinophilia: What is in a name? Clin Gastroenterol Hepatol 2014 Jul 30. pii: S1542-3565(14)01080-5. [ Links ]
45. Straumann A, Spichtin HP, Grize L, Bucker KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: A follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003;125:1660-9. [ Links ]
46. Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013;145:1230-6. [ Links ]
47. Straumann A, Schoepfer A. Update on basic and clinical aspect of eosinophilic oesophagitis. Gut 2014;63:1355-63. [ Links ]
48. Dohil R, Newbury RO, Aceves S. Transient PPI responsive esophageal eosinophilia may be a clinical sub-phenotype of pediatric eosinophilic esophagitis. Dig Dis Sci 2012;57:1413-9. [ Links ]
49. Schroeder S, Capocelli KE, Masterson JC, Harris R, Protheroe C, Lee JJ, et al. Effect of proton pump inhibitor on esophageal eosinophilia. J Pediatr Gastroenterol Nutr 2013;56:166-72. [ Links ]
50. Molina-Infante J, Martinek J, Martinez-Alcala C, Krajciova J, Moawad FJ, Dellon ES. Long-term efficacy of ppi therapy in patients with ppi-responsive esophageal eosinophilia: An International Multicenter Study. Gastroenterology 2014;146(Supl. 1):S-393. [ Links ]
![]() Correspondence:
Correspondence:
Javier Molina-Infante.
Department of Gastroenterology.
Hospital San Pedro de Alcántara.
C/ Pablo Naranjo, s/n.
10003 Cáceres, Spain
e-mail:
xavi_molina@hotmail.com
Received: 07-10-2014
Accepted: 27-10-2014