My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.3 Madrid Mar. 2015
Validity and reliability of the minimum basic data set in estimating nosocomial acute gastroenteritis caused by rotavirus
Validez y fiabilidad del conjunto mínimo básico de datos en la estimación de la gastroenteritis aguda nosocomial por rotavirus
Olga Redondo-González
Research Support Unit. Hospital General La Mancha Centro. Alcázar de San Juan, Ciudad Real, Spain.
Social and Cardiovascular Epidemiology Research Group. School of Medicine, Universidad de Alcalá. Alcalá de Henares, Madrid. Spain
ABSTRACT
Introduction: Rotavirus is the principal cause of nosocomial acute gastroenteritis (NAGE) under 5 years of age. The objective is to evaluate the validity and reliability of the minimum basic data set (MBDS) in estimating the NAGE caused by rotavirus (NAGER) and to analyze any changes during the three years that the Rotarix® and Rotateq® vaccines were used in Spain.
Material and methods: A descriptive, retrospective study was carried out in the University Hospital of Guadalajara (UHG) (Spain) between 2003-2009 using the MBDS, positive microbiological results for rotavirus (PMRs), and medical histories. Three methods of estimation were used: 1) An ICD-9-CM code 008.61 in the secondary diagnosis fields (DIAG2) of MBDS; 2) method 1 and/or PMRs with a current or recent hospitalization; and 3) the reference method or method 2 contrasted with patient medical histories. The validity of methods 1 and 2 was determined -sensitivity, specificity, predictive values and likelihood ratios (LRs)-, along with their agreement with method 3 (Kappa coefficient). In addition, the incidence rate ratio between the NAGER rate in 2007-2009 (commercialization period of both vaccines) was calculated with respect to 2003-2005 (pre-commercialization period).
Results: Method 1 identified 65 records with a DIAG2 of 008.61. Method 2 found 62 probable cases, and the reference method, 49 true cases. The sensitivity of the MBDS was 67 %, the positive predictive value was 51 %, and both negative LR (LR-) and reliability were moderate (LR- 0.33, Kappa coefficient 0.58). During 2007-2009, the NARGE decreased by 5 cases per 103 hospitalizations by 9 per 104 days of hospitalization. Method 2 overestimated both the decline in incidence by 2 per 103 hospitalizations and the decreased risk per day of stay by 10 %. The MBDS found no differences between the two three-year periods, but, like method 2, showed an excellent level of diagnostic evidence (LR+ 67).
Conclusion: The MBDS taken together with microbiological results, is more exact, safer and more reliable than the MBDS alone in estimating NAGER; and more useful in ruling out it. Nevertheless, the MBDS alone may be used to estimate and compare such disease in contexts with different prevalences.
Key words: Rotavirus infections. Rotavirus vaccines. Gastroenteritis. Hospital infections. Medical registries. Spain.
RESUMEN
Introducción: la gastroenteritis aguda nosocomial por rotavirus (GEANOR) representa la principal causa de GEA nosocomial en menores de 5 años. El objetivo es evaluar la validez, fiabilidad y verosimilitud del conjunto mínimos básico de datos (CMBD) para estimarla y analizar posibles cambios en el primer trienio de comercialización de las vacunas Rotarix® y Rotateq® en España.
Material y métodos: estudio descriptivo-retrospectivo en el Hospital Universitario de Guadalajara en 2003-2009 mediante el CMBD (altas neonatales y pediátricas), los resultados positivos para rotavirus de microbiología (RPM) y las historias clínicas. Los métodos de estimación fueron: 1 (CIE9-MC 008.61 en los campos de diagnóstico secundario del CMBD); 2 (método 1 y/o RPM con ingreso actual-reciente); y método 3 o referencia (método 2 contrastado con las historias clínicas). Se determinó la validez de los métodos 1 y 2 y su concordancia con el método 3 (índice kappa). También, las razones de verosimilitud (LHR) de estos métodos. Además, se calculó la razón de tasas de incidencia de 2007-2009 respecto a 2003-2005.
Resultados: se encontraron 65 registros con DIAG2 008.61 y 62 casos probablemente nosocomiales por el método 2, cuyas historias clínicas fueron revisadas junto con 11 registros posiblemente nosocomiales. La sensibilidad del CMBD resultó del 67 %, la seguridad del 51 % y su fiabilidad y LHR negativo moderados (índice kappa 0,58 y LHR- 0,33). En 2007-2009, respecto a 2003-2005, la enfermedad se redujo en 5 casos por 103 ingresos y en 9 por 104 días de estancia. El método 2 sobreestimó el descenso en 1 por 103 ingresos y el descenso de riesgo por día de estancia en un 10 %. El CMBD no encontró diferencias intertrienios pero presentó al igual que el método 2, un excelente nivel de evidencia diagnóstica (LHR+ 67).
Conclusión: el CMBD junto a la microbiología es más exacto, seguro y consistente que el CMBD per se en la estimación de la GEANOR, y también más útil para descartarla. Sin embargo, el CMBD por sí mismo podría ser utilizado en la estimación de la infección nosocomial en contextos con diferentes prevalencias.
Palabras clave: Infecciones por rotavirus. Vacunas contra rotavirus. Gastroenteritis. Infección hospitalaria. Registros médicos. España.
Abreviations
AGE: Acute gastroenteritis;
NAGE: Nosocomial acute gastroenteritis;
NAGER: Nosocomial acute gastroenteritis caused by rotavirus;
MBDS: Minimum basic data set;
DIAG2: Secondary diagnosis fields;
DIAG1: Principal diagnosis fields;
UHG: Hospital Universitario de Guadalajara (Spain);
PMRs: positive microbiology results for rotavirus;
95 % CI: 95 % confidence interval;
PPV: Positive predictive value;
NPV: Negative predictive value;
IR: Incidence rate;
LR: Likelihood ratio;
IRR: Incidence rate ratio;
IQR: Interquartile range.
Introduction
Nosocomial acute gastroenteritis (NAGE) caused by rotavirus (NAGER) occurs in 5 % in hospitalized children, leading to increased hospital stays and the use of extra hospital resources (1-3). Rotavirus is the main cause of NAGE in children under the age of 5 years (31-87 %) (4-7), with 70 % of cases occurring in children under 1 year of age (8). The seasonal pattern coincides with the winter peak of other pediatric viral infections (4), although this period is extended with respect to that of community-acquired rotavirus, starting in the autumn and finishing in the early spring (6).
NAGER becomes full blown after a 3-day incubation period (3). Twenty to forty percent of all cases are asymptomatic or subclinical (6), which, together with the great stability of rotavirus, contributes to contagion and the low efficacy of control measures (9). In Europe, nosocomial cases constitute 14-51 % of all rotavirus infections in children under 5, with an incidence of 0.1 to 2.8 per every 103 hospital admissions (newborns: 4-7.3 per 103). The additional costs for treating the infection can range from 367 to 1,837 euros per case (4-6,10-12). The differences in incidence between countries reflect the heterogeneity of studies conducted on this disease, making comparisons difficult (10,11,13,14). The same occurs between Spanish regions. Few studies have been carried out on NAGER in Spain (3,4,6,8). In Europe as a whole, the average incidence rate is around 4.6 per 104 days of hospitalization (range: 0.1-6.8 per 104), which lengthens the average hospital stay between 4 and 12 days (13). The attack rate of symptomatic rotavirus infection can reach up to 56 % in outbreak periods (14,15).
The Rotarix® and Rotateq® vaccines were first marketed in Spain in July, 2006, and February, 2007, respectively (4). At the time, they were not included in the Spanish vaccination schedule (16), nor were they added in 2013 (17) as they were not considered cost effective (18). However, given the morbidity of the disease and the increased burden on the healthcare system, vaccination is generally recommended (19). The new vaccines are considered safe, with a 5-10 times lower risk of intussusception (20) than that associated with Rotashield® in the past. With a universal vaccination program and assuming 90 % coverage, it is estimated that up to 58 % of NAGER cases in Spain could be avoided over a 5-year period (21).
The recording of rotavirus in hospital information systems has been described as highly deficient (5), partly due to the elevated incidence of asymptomatic cases of NAGER (22). Furthermore, the attack rate of the disease after discharge may be as high as 16 %, and if these cases are mild or asymptomatic, they generally remain unreported. Nevertheless, the minimum basic data set (MBDS) and other similar tools have been used to assess the incidence of NAGER (8,10). It is uncertain whether the secondary diagnoses fields (DIAG2) in the MBDS constitute a valid method for identifying the disease, as they give no information of the time between hospitalization and onset; moreover, some microbiological diagnostic results are administered after hospital discharge. In a similar vein, the MBDS contains cases categorized as the principal diagnosis field (DIAG1), which in reality correspond to infections acquired during a prior hospitalization. Likewise, the consistency of DIAG2 in always giving equivalent results (diagnostic agreement) has not been determined. Although the code system is well-established, variability still arises due to the different experience of those doing the actual coding and how the clinicians filled out medical histories at discharge.
The main objective of this study is to assess the validity and reliability of the DIAG2 of the MBDS in estimating NAGER. Also, to analyze any possible changes in incidence during the first three-year period that the new vaccines against rotavirus were commercially available in Spain.
Material and methods
This is a descriptive, retrospective study carried out at the Hospital Universitario de Guadalajara (UHG, Spain) between 2003 and 2009. Three data sources were used:
1. The MBDS of the UHG: The mandatory registry for hospital discharges for any reason in the pediatric and neonatal wards.
2. The computerized Modulab® registry of the Microbiology laboratory of the UHG: Positive microbiology results for rotavirus (PMRs) found with a rapid and simultaneous detection immunochromatographic kit for rotavirus and adenovirus (VIKIA Rota-Adeno®, bioMérieux). This method has a reproducibility of 100 %, a sensitivity of 92 % (95 % confidence interval (95 % CI) 84-99 %), and a specificity of 91 % (95% CI 78-100 %). Its positive predictive value (PPV) is 96 % (90-100 %) whereas its negative predictive value (NPV) is 83 % (67-98 %).
3. Medical histories from the central archives of the UHG, of the probable and possible nosocomial cases as defined below in methods 2 and 3, respectively.
This project was approved by the hospital's Ethics Committee for Clinical Research of Guadalajara (Spain).
Definition of the variable of interest and the estimation methods
NAGER was defined as "the development of symptoms 72 hours after hospital admission and up to 72 hours after discharge" (3). The estimation methods were defined according to the following data selection criteria:
1. Method 1: Registry entries with a code of The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) 008.61 (rotavirus) in any of the DIAG2 of the MBDS, where the DIAG1 field was something other than 558.9 (unspecified AGE), 787.03 (vomiting), or 787.91 (diarrhea).
2. Method 2: Method 1 combined with PMRs coinciding with a current or recent hospitalization (within the last 3 days) according to the MBDS. The PMRs were joined with the MBDS, using the medical history numbers of patients as the crossover tool. We searched for codes of 008.61, 558.9, 787.03, 787.91, or 780.06 (fever) in the DIAG1 or the DIAG2 coinciding with a PMR.
Probable nosocomial cases were defined as episodes (registry entries) in the MBDS with:
- A DIAG1 other than 008.61 and a DIAG2 of 008.61 without PMR.
- A DIAG1 other than 008.61 and a DIAG2 of 008.61, 558.9, 787.03, or 787.91 with PMR 72 hours after hospitalization or within the first 72 hours after discharge. Also, as an exception, newborns with PMR occurring ≤ 72 hours after hospitalization.
- A DIAG1 of 008.61, 558.9, 787.03, or 787.91 and previous hospitalization with a DIAG1 other than 008.61 and a discharge within 72 hours preceding the second admission, besides a PMR from 72 hours after the first hospitalization or during the second.
3. Method 3 (reference or "clinical method"): Method 2 contrasted with patient medical histories. To find true nosocomial cases, we reviewed not only the medical histories of those cases categorized by method 2 as probable nosocomial cases, but also those of the possible nosocomial cases, that is, registries with a DIAG1 of 008.61, 558.9, 787.3, or 787.91 and a prior hospitalization with a DIAG1 other than 008.61 without PMRs. A data collection sheet based on one used in a prior study on NAGER (6) was elaborated to this end.
Cases that were ruled out as being true nosocomial cases were categorized as false nosocomial cases or as community-acquired cases, both of which would be false positives according to method 2.
Statistical analysis
Frequency indicators were calculated for NAGER cases in the pediatric and neonatal wards, globally and by department, by age group, by year of study period, and by the two three-year periods (2003-2005 and 2007-2009), with their corresponding 95 % CI, with each method described. Prior to calculating incidence rates (IRs), lengths of hospital stays were previously estimated.
We then determined the sensitivity and specificity (internal validity) for methods 1 and 2, along with external validity: The PPVs and NPVs (performance or safety) and positive and negative LRs (LHR+ and LHR-) (plausibility). In addition, Chamberlain's percentage of positive agreement (% PA) was calculated (23). We lastly estimated the kappa index values to determine the agreement of methods 1 and 2 with regard to the clinical method, using the Altman scale (24) in interpreting the data thus obtained.
The seasonal pattern was described by means of the monthly distribution of the number of cases. To compare the prevalence of the disease among different pediatric age groups, three-year study periods, and estimation methods, the chi-square test was used. We compared the IR of NAGER in the three-year study period when Rotarix® and RotaTeq® were simultaneously available in Spain (2007-2009) with the previous three-year period (2003-2005), by calculating the incidence rate ratios (IRRs) with each method. The year 2006 was considered to be a transitional period, as only Rotarix® was then commercially available in Spain.
All analyses were carried out with SPSS 15.0 software and the Epi InfoTM 7 application for Windows. A p value of 0.05 indicated statistical significance.
Results
Between 2003 and 2009, a total of 9,602 children were hospitalized for any reason in the UHG, 21 % in the neonatal ward. A DIAG1 of 008.61 appeared for 381 of the children hospitalized, with another 65 admissions listing it as a DIAG2 (Fig. 1). According to method 1, seven of every 103 children hospitalized at the UHG in the 2003-2009 period had acquired NAGER, with a prevalence 6 ‰ higher in neonatal patients than in pediatric patients (Table I).
No registry entries coded as 780.06 appeared as a DIAG1 or DIAG2 concomitantly with PMR. Two of the 65 entries in the MBDS with a DIAG2 of 008.61, both for pediatric admissions, had no PMRs. We found 37 registry entries with a DIAG1 other than 008.61 with PMR 72 hours after hospitalization, 36 with a DIAG2 of 008.61, and 1 with a DIAG2 of 558.9, belonging to a pediatric patient (Fig. 1). Of the 36 patients with a DIAG2 of 008.61, 2 of the 23 entries coming from the pediatric ward corresponded to the same patient, but they were episodes from different years. In addition, we found 5 entries with a DIAG2 of 008.61 that corresponded to newborns with PMRs obtained ≤ 72 hours after hospitalization. Furthermore, there were 16 entries with a DIAG1 of 008.61 and 2 with a DIAG1 of 558.9, all with PMRs and a prior hospitalization for a DIAG1 other than rotavirus, who had been discharged in the previous 72 hours, all from the pediatric ward (Fig. 1). The final tally of probable nosocomial cases was 62, all of which were under 5 years of age and 18 of which were neonatal patients. According to method 2, seven of every 103 children hospitalized between 2003 and 2009 had acquired NAGER, with a prevalence a 3 ‰ higher in neonatal patients (Table I).
We identified 11 entries with a DIAG1 of 008.61, 558.9, 787.3, or 787.91 who had been discharged within 72 hours prior to being readmitted, but without PMRs; these were classified as possible nosocomial cases and were added to the 62 probable nosocomial cases (Fig. 1). In total, 73 medical records were reviewed. The 2 probable nosocomial cases with a DIAG2 of 008.61 and no PMR turned out to be coding errors and were reclassified as false nosocomial cases. Of the 37 entries in the MBDS with a DIAG1 other than 008.61 and PMRs 72 hours after hospitalization, 6 turned out to have been community-acquired and 31, including all the neonatal cases, were true nosocomial cases. The 5 newborns with PMRs obtained ≤ 72 hours after hospitalization were also true nosocomial cases.
Of the 18 hospitalizations for a DIAG1 of 008.61 or 558.9, with PMRs and a prior hospitalization for a DIAG1 other than rotavirus, and with a discharge in the previous 72 hours, 5 turned out to have been community-acquired (of those, the 2 categorized as 558.9) while 13 were true nosocomial cases. The 11 cases thought to be possible nosocomial cases all had medical histories that were incompatible with NAGER and were thus classified as false nosocomial cases. We were thus able to categorize 67.1 % (n = 49) of the medical histories reviewed as true nosocomial cases (Fig. 1). Of these, 36.7% (n = 18) occurred in neonates (Table I). Overall, 51 % of these true nosocomial cases occurred in male patients, 90 % (n = 44) occurred under the age of 2, a 35 % (n = 17) occurred in newborns; and 30 % (n = 15, all pediatric patients) had been discharged within the previous 72 hours. According to the reference method, 5 of every 103 children hospitalized in the 2003-2009 period had contracted NAGER in hospital, with a 5 ‰ higher prevalence in neonatal patients (Table I) and a post-discharge attack rate of 2 ‰. A statistically significant difference was observed between the calculation method used and the prevalence of NAGER (p = 0.001).
The sensitivity and PPV of method 1 in detecting NAGER were 67 % and 51 %, respectively, while for method 2 they were 100 % and 79 %, respectively. The LR+ and LR- of method 1 were 67 and 0.33, respectively, in contrast with 100 and 0 for method 2. The degree of positive agreement with respect to the reference method was 52 % greater for method 2 (Table II). The reliability of the MBDS was greater when contrasted with the microbiology results (kappa indices of 0.58 vs. 0.88) (Table II).
For neonatal patients, the mean age of children with NAGER was 0 days (minimum = 0, maximum = 29) while for pediatric patients it was 10 months (minimum = 1, maximum = 89). The cumulative percentage in children under 1 year of age was 75.5 %, with a greater incidence between 7 and 11 months (Fig. 2). The illness was significantly associated with age, with children under 2 years of age having a 9-fold risk of infection by rotavirus compared to children being 2 and over (p < 0.001). The main reason for hospitalization in neonatal patients was "preterm or prematurely born newborn" (73 %) while for pediatric patients it was for bronchiolitis/bronchospasms (32 %) and lower respiratory tract infections (19 %).
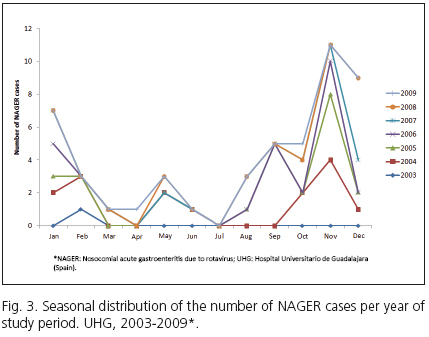
Cases of NAGER followed a seasonal pattern similar to that of AGE caused by community-acquired rotavirus, with higher peaks in the second half of autumn and the first half of winter (November-January), when a little over half of all the annual cases occurred, along with other minor peaks in spring (Fig. 3). The greatest amount of cases occurred in November. There were also summer outbreaks in 2005 and 2007.
Between 2003 and 2009, the mean number of NAGER cases was 4.14 per 103 hospitalizations per year (Table I) The incidence rate was similar when calculated with method 2, and was much higher when calculated with method 1. The difference between methods 1 and 2 with respect to the reference method was found mostly in pediatric cases, where the two methods estimated 2 and 1 cases more per 103 hospitalizations/year, respectively, than the reference method (Table I). By the reference method, incidence reached 2 maximum peaks, one in 2004 and another lesser peak in 2007. Incidence decreased in 2008 and again, albeit not significantly, in 2009 (Fig. 4). The annual incidence distribution reflects a similarity among the different methods for assessing seasonal evolution (Fig. 4). The mean hospital stay for all hospitalizations in 2003-2009 was 4 days (interquartile range [IQR]= 3.7-4.3): Three (2.5-3.4) for pediatric cases and 7.9 (7.3-8.5) for neonates. For patients with NAGER, it was 9 days (IQR = 5-20): 7 (4-9) for pediatric cases and 25 (16-37.3) for neonates. In premature infants, it was 29 days (IQR = 20-39; minimum = 15, maximum = 44).
In the period between 2007 and 2009 there was an overall decrease of 5 cases for every 103 hospitalizations (approx. 2.8 ‰ less frequent) with respect to the 2003-2005 period (p < 0.001). When measured with method 2, this decrease was even greater, at 3.6 ‰ (p < 0.001) (Table III). In contrast, no significant differences between the two three-year periods were observed when calculated with method 1 (p = 0.33). When the reference method was used, the IR went down by 9 cases for every 104 days of hospital stay (IRR2007-2009/2003-2005 = 0.55; 95 % CI 0.54-0.56; p < 0.001). The IRR between the two three-year periods with method 2 was 0.45 (95 % CI 0.44-0.46); when measured with the MBDS, it was 1.08 (95 % CI 1.06-1.10) (Table III).
Discussion
The DIAG2 taken from the MBDS detected 67 % of NAGER cases during the 2003-2009 period, with a safety of 51 % and a moderate power of agreement with respect to the clinical method. In contrast, the MBDS taken together with microbiology results produced maximum detection, with a 28 % higher safety and very good agreement. Although both methods showed an excellent level of diagnostic evidence (LR+ > 10), method 1 was only moderately effective for ruling out the illness (LR- = 0.33) when it was not detected. Throughout the study period, the mean incidence of NAGER showed high variability, with a range of 2.7-7.8 cases per 103 hospitalizations/year. This was also true for the mean IR, which ranged from 7.0 to 77.7 cases for every 104 days of hospital. The disease was 56 % more frequent in neonatal than in pediatric patients. On average, the hospital stay for children who contracted NAGER in the hospital was 4 days longer in pediatric cases and 17 days more for neonates. In the 2007-2009 period, the illness was almost 2 times less prevalent compared to 2003-2005, with the risk of contracting the illness per day of hospital stay decreasing almost by half. While in the three-year period in which privately funded vaccines were available, the number of cases/year showed a decrease of 55 %, universal vaccination programs in Europe in the same time period showed a prevention of up to 72 % of cases/year (25).
The selected study period was selected for convenience, to evaluate the efficacy of vaccines before 2010, when the Spanish Agency for Medicines and Health Products interrupted the supply of Rotarix® and Rotateq® because of the detection of porcine circovirus in March and June of that year, respectively (18). Maximum vaccine coverage, despite the fact that the vaccines were not subsidized by the Spanish healthcare system, would have thus occurred when the drugs were first made available, from 2007 to 2009, a time when pediatricians were recommending the health benefits of vaccination during well child visits, thereby promoting their commercialization. Although in November, 2010, Rotateq® was re-released into the market, Rotarix® is still unavailable in Spain (17). We then decided to compare the 2007-2009 period with an immediately preceding period of equal length, leaving out the year 2006, as explained above in the methods section.
Overall, the MBDS per se overestimated the incidence by 3 cases for every 103 hospitalizations and the mean IR by 6 cases for every 104 days of hospital stay. Besides, it did not detect the decrease during the 2007-2009 period. Together with microbiology results, the MBDS did indicate this decrease, although it overestimated it by 2 cases per 103, as well as the decrease in risk per day of hospital stay by 10 %. It is difficult to compare the incidence found in other Spanish studies due to the heterogeneity in the designs and methodologies used (3,4,6,8). In this context, the variability of the assessment methods would be an important source of error (26). Furthermore, the majority of previous studies have been retrospective, which supposes a lack of follow-up in the 72 hours after discharge and the exclusion of mild or asymptomatic cases (4,8,10). Previous studies based solely on hospital discharge data with the specific code of CIE-9-MC 008.61 have been shown to underestimate the real burden of the disease (4). To evaluate the validity and reliability of the MBDS, we took the patients' medical histories as a reference, as they contain more complete information on patient hospitalizations and, together with the PMRs, they offer more accurate data. The fact is, as mentioned above, that almost a third of identified as true nosocomial cases had been discharged less than 72 hours before being readmitted with NAGER as their DIAG1; that is, the disease became symptomatic after the first discharge.
The difficulty in comparing different studies in this field is an even greater challenge in the European context, with discrepancies among public health systems and different epidemiological patterns (5,10). The changes observed between the two three-year periods under study, as well as any comparisons made between national and international results must be taken with caution given the low number of cases of NAGER and the low statistical power of the sample.
The UHG was an ideal place to carry out this study because its microbiology lab is a place of reference throughout the region. Feces sample collection to check for rotavirus in children is routinely carried out if there is any suspicion of AGE whenever any symptoms of the disease are observed. Because of this, the date of the microbiology results was taken to be the start of symptomatic AGE in order to estimate probable nosocomial cases, possible nosocomial cases, and true nosocomial cases (Fig. 1). For those entries with PMRs within the first 72 hours after hospitalization, a community origin was assigned. Nevertheless, a PMR after this period did not necessarily indicate a nosocomial origin since there is sometimes a time lapse between the start of symptoms and a PMR. These cases were thus defined as probable nosocomial cases. Those in which a PMR was obtained within 72 hours of discharge were considered to be nosocomial, but even after this period, we could not entirely rule out a nosocomial origin since in cases with mild symptoms, outpatient feces sampling may be delayed or not performed. In order not to lose these cases, we reviewed all medical histories with PMRs obtained up to one month after hospital discharge with a DIAG2 of 00.861, and/or 558.9, and/or 787.03, and/or 787.91. As mentioned above, we identified 37 cases with PMRs on the third day after discharge, 36 with a DIAG2 of 008.61 and one with a DIAG2 of 558.9 (Fig. 1). In one month follow-up of hospitalizations due to rotavirus, readmitted patients usually are within three days after the initial discharge (27). Still, this would not have identified mild or asymptomatic cases after discharge with no DIAG2 entries of 558.9, and/or 787.03, and/or 787.91, and/or without microbiology sample collection. This has prompted a call to include in future a new label of "present on admission" (POA), which would accompany each diagnostic code to differentiate diagnoses made at the time of hospital admission (POA = Y, yes) from those appearing during the actual hospital stay (POA = N, no).
The mean incidence of NAGER calculated through the DIAG2, namely 6.90 per 103, is higher than the national average (obtained by the same means) of 4.50 per 103 (8). Although the reliability of the MBDS is only moderate, this comparison reveals a high incidence of NAGER in the UHG. Moreover, the mean IR of 11.07 per 104 (IR: 7-77.72) obtained with the clinical method is double that described for Western Europe, which is 4.6 per 104 (range: 0-6.8 per 104) (13). Indeed, the mean IR reported for Europe as a whole is similar to those reported in prospective studies (1,11). In this context, the incidence observed in the UHG of 4.14 per 103 is quite low if we compare it with prospective studies carried out in Spain (3,6,28). This difference may be due to the aforementioned loss of mild or asymptomatic cases, especially in the absence of follow-up period, as well as to the loss of symptomatic cases that remain unclassified as such in the MBDS because they were not properly entered in the medical histories or for lack of microbiology results. This could be a possible source of selection bias in our study. Even in prospective studies which only record results of symptomatic infection, the observed prevalence is higher than in our study (3). Taking these considerations into account, the main limitation of this study is the type of information in the MBDS. Indeed, it has been estimated that post-discharge appearance of NAGER occurs in up to 11 cases for every 103 hospitalizations (29). Even so, it would be necessary to perform feces analyses both before and after discharge to identify asymptomatic cases (30).
In addition to all the aforementioned, and with respect to the difficulty in making comparisons, it must be remembered that the incidence is influenced by the seasonal period followed (epidemic season vs. complete year) and the age range of the patients (14). Surveillance studies have determined a combined incidence of NAGER that can reach up to 8.1 cases during epidemic months (95 % CI 6.4-9.9) (31).
The use of medical histories as a reference also presents several limitations such as interviewer bias. The form in which each clinic records the beginning and the course of digestive symptoms in each patient's medical history may differ depending on his or her particular interest the pathology observed or the severity of its evolution. For this reason, we chose to also review the nursing records, in which the number and consistency of stools is recorded with greater accuracy, as is the presence of vomiting. Further, as the medical records were all reviewed by the same person, interobserver agreement could thus not be calculated, which could produce observer bias (erroneous differential classification of disease). We attempted to minimize this by elaborating a systemized data collection form. But this same error could occur in the process of codification, depending on the experience of those applying the disease codes.
To date, no studies have specifically evaluated the efficacy of the MBDS as a surveillance system for NAGER, although it has been used to monitor other nosocomial infections such as surgical site infections, catheter-associated urinary tract infection, central line-associated bloodstream infection, ventilator-associated pneumonia/events, postprocedure pneumonia, as well as infections caused by bacteria associated with hospital health care (methicillin-resistant Staphylococcus aureus and Clostridium difficile) (32). It has also been used to determine the prevalence of nosocomial infections (33) and its agreement with other data sources in detecting cancer (34), emergency pathologies (35), postoperative risk factors and secondary effects (36), cerebrovascular trauma (37), and surgical mortality (38) have been assessed. As in our study, other researchers have found a moderate sensitivity and high specificity of the MBDS in detecting Clostridium difficile and surgical site infection (32). Due to the low incidence of hospital infection, the PPV of registries like the MBDS are probably low.
This is also the first published analysis of the effects of the commercialization of both vaccines against rotavirus on NAGER incidence in Spain. In countries with a nation-wide immunization schedule, the indirect benefits on the non-vaccinated public have previously been demonstrated (39,40), albeit not at the intrahospital level. Nevertheless, the decrease observed in the three-year study period after the vaccines had been made available may be due to herd immunity as well as to the "hand washing program" promoted by the UHG since 2008, a strategy that has proven effective in lowering the communicability of the virus within the hospital, which is found on the hands of 77 % of hospital health workers (5,41). Still, only 37 % of NAGER cases that we identified had actually been isolated, all of them in neonatal patients. Other researchers have estimated that the disease is isolated only in 50 % of patients (14).
This study has analyzed for the first time the validity and reliability of the DIAG2 of the MBDS in estimating NAGER. The clinical method ruled out 25 % of the cases categorized by this system. The MBDS, taken together with microbiology results, equivalent to the Microbiology Information System, is more exact, safer and more reliable than the MBDS alone in estimating NAGER; and better at ruling out it. Nevertheless, because it offers a high level of diagnostic evidence, method 1 may be used to estimate nosocomial infection in contexts with various prevalences, always with caution. In this sense, future studies with larger sample sizes should be undertaken to bolster these findings. We should raise awareness among healthcare professionals about the importance of proper recording of clinical variables to improve the coding in Healthcare Information Systems. If we cannot validly and efficiently assess the quality of care, it will be impossible to design strategies to improve monitoring and control of nosocomial infections. The implementation of a specific code to register this pathology in the MBSD would be an important step forward in this regard.
Acknowledgements
I would like to thank Antonio Clemente of the healthcare information department of the UHG Hospital Admissions and Documentation Service for allowing me to access to the MBDS and for his helpful explanations. I am also grateful to Carmen Gimeno of the UHG Microbiology Lab for extracting the positive results for AGE caused by rotavirus during the study period. Thanks are also due to my colleagues in the Research Unit at the La Mancha Centro Hospital for assisting with the final revision of this manuscript. Finally, I would like to thank the technicians at the Central Archives of the UHG, who provided me with the medical histories necessary for carrying out this research as well as the MBDS coders at the UHG for their help in responding to my questions.
References
1. Verhagen P, Moore D, Manges A, Quach C. Nosocomial rotavirus gastroenteritis in a Canadian paediatric hospital: Incidence, disease burden and patients affected. J Hosp Infect 2011;79:59-63. [ Links ]
2. Gutiérrez-Gimeno MV, Martin-Moreno JM, Díez-Domingo J, Asensi-Botet F, Hernández-Marco R, Correcher-Medina P, et al. Nosocomial rotavirus gastroenteritis in Spain: A multicenter prospective study. Pediatr Infect Dis J 2010;29:23-7. [ Links ]
3. Román Riechmann E, Wilhelmi de Cal I, Cilleruelo Pascual ML, Calvo Rey C, García García ML, Sánchez-Fauquier A. Nosocomial gastroenteritis and asymptomatic rotavirus and astrovirus infection in hospitalized children. An Pediatr (Barc) 2004;60:337-43. [ Links ]
4. Hernández Pezzi G, Varela Martínez MC. Vigilancia epidemiológica de las gastroenteritis agudas víricas. Laia Alemany Vilches L, Moraga Llop F, García Jiménez S. En: Bellido Blasco JB, coordinador. 6a Monografía de la Sociedad Española de Epidemiología. EMISA 2007;7:65-77. [ Links ]
5. Gleizes O, Desselberger U, Tatochenko V, Rodrigo C, Salman N, Mezner Z, et al. Nosocomial rotavirus infection in European countries: A review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J 2006;25(1 Supl.):S12-21. [ Links ]
6. Gutiérrez Gimeno MV. Gastroenteritis aguda por rotavirus en población infantil ingresada en unidades de lactantes de Valencia; 2009 (Internet). (Acceso: 24 febrero 2013). Disponible en: http://rodrigo.uv.es/handle/10550/15899. [ Links ]
7. Puacz E, Cwikla S, Piasecka-Twaróg M. Analysis of norovirus and rotavirus infections of patients hospitalized in the General Specialist Hospital in Lublin. Retrospective studies. Med Dosw Mikrobiol 2013;65:57-64. [ Links ]
8. Gil-Prieto R, San Martín M, de Andrés AL, Álvaro-Meca A, González A, de Miguel AG. Hospital-acquired rotavirus infections in Spain over a ten-year period (1998-2007). Hum Vaccin 2009;5:748-53. [ Links ]
9. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 2006;6:130. [ Links ]
10. The Pediatric Rotavirus European Committee (PROTECT). The paediatric burden of rotavirus disease in Europe. Epidemiol Infect 2006;134:908-16. [ Links ]
11. Stefkovicová M, Simurka P, Juracková L, Hudecková H, Mad'ar R. Nosocomial rotaviral gastroenteritis in paediatric departments. Cent Eur J Public Health 2008;16:12-6. [ Links ]
12. Bentama I, Soussi I, Ghanimi Z, Riane S, Tligui H, Mdaghri Alaoui A, et al. Epidemic of nosocomial infection by rotavirus in a neonatology service. Rev Med Brux 2012;33:519-24. [ Links ]
13. Ogilvie I, Khoury H, Goetghebeur MM, El Khoury AC, Giaquinto C. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: A scoping review. BMC Infect Dis 2012;12:62. [ Links ]
14. Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009;123:e393-e400. [ Links ]
15. Bentama I, Soussi I, Ghanimi Z, Riane S, Tligui H, Mdaghri Alaoui A, et al. Epidemic of nosocomial infection by rotavirus in a neonatology service. Rev Med Brux 2012;33:519-24. [ Links ]
16. Consejo Interterritorial del Sistema Nacional de Salud. Calendario de Vacunaciones recomendado (2007). (Internet) (Acceso 20 abril 2012). Disponible en: http://www.msps.es/ciudadanos/proteccionSalud/infancia/vacunaciones/programa/vacunaciones.htm. [ Links ]
17. Consejo Interterritorial del Sistema Nacional de Salud. Calendario común de vacunación infantil (2013). (Internet) (Acceso 23 abril 2013). Disponible en: http://www.msc.es/gabinetePrensa/notaPrensa/pdf/21.03210313201619746.pdf. [ Links ]
18. Imaz Iglesia I, Cornejo Gutiérrez AM, Rubio González B, González Enríquez J. Análisis coste-utilidad de la introducción de la vacunación universal frente al rotavirus en España. Madrid: AETS - Instituto de Salud Carlos III, Madrid; 2011. [ Links ]
19. Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Barrio Corrales F, Cilleruelo Ortega MJ, Corretger Rauet JM, et al. Calendario de vacunaciones de la Asociación Española de Pediatría: recomendaciones 2013. An Pediatr 2013;78:59.e1-27. [ Links ]
20. Parez N, Giaquinto C, Du Roure C, Martinon-Torres F, Spoulou V, Van Damme P, et al. Rotavirus vaccination in Europe: Drivers and barriers. Lancet Infect Dis 2014;14:416-25. [ Links ]
21. Diez-Domingo J, Suriñach NL, Alcalde NM, Betegón L, Largeron N, Trichard M. Burden of paediatric rotavirus gastroenteritis (RVGE) and potential benefits of a universal Rotavirus vaccination programme with a pentavalent vaccine in Spain. BMC Public Health 2010; 10:469. [ Links ]
22. Gianino P, Mastretta E, Longo P, Laccisaglia A, Sartore M, Russo R, et al. Incidence of nosocomial rotavirus infections, symptomatic and asymptomatic, in breast-fed and non-breast-fed infants. J Hosp Infect 2002;50:13-7. [ Links ]
23. Carrasco JL, Jover L. Métodos estadísticos para evaluar la concordancia. Med Clin (Barc) 2004;122(Supl. 1):28-34. [ Links ]
24. Altman DG. Practical statistics for medical research. Chapman & Hall/CRC; 1991. [ Links ]
25. Abraira V. Errores en las mediciones y clasificaciones clínicas: precisión y validez. (Internet). (Acceso: 29 octubre 2012). Disponible en: http://www.hrc.es/bioest/Intro_errores.html. [ Links ]
26. Zlamy M, Kofler S, Orth D, Würzner R, Heinz-Erian P, Streng A, et al. The impact of Rotavirus mass vaccination on hospitalization rates, nosocomial Rotavirus gastroenteritis and secondary blood stream infections. BMC Infect Dis 2013;13:112. [ Links ]
27. Pockett RD, Campbell D, Carroll S, Rajoriya F, Adlard N. Rotavirus, respiratory syncytial virus and non-rotaviral gastroenteritis analysis of hospital readmissions in England and Wales. Acta Paediatr 2013;102:e158-63. [ Links ]
28. Marc E, Biscardi S, Soulier M, Lebon P, Gendrel D. Nosocomial rotavirus infections in a pediatric unit: Surveillance during four successive winters. Med Mal Infect 2007;37:61-6. [ Links ]
29. Buettcher M, Heininger U. Prospective surveillance of nosocomial viral infections during and after hospitalization at a university children's hospital. Pediatr Infect Dis J 2010;29:950-6. [ Links ]
30. Borrows CL, Turner PC. Seasonal screening for viral gastroenteritis in young children and elderly hospitalized patients: Is it worthwhile? J Hosp Infect 2014;87:98-102. [ Links ]
31. Bruijning-Verhagen P, Quach C, Bonten M. Nosocomial rotavirus infections: A meta-analysis. Pediatrics 2012;129:e1011-9. [ Links ]
32. Goto M, Ohl ME, Schweizer ML, Perencevich EN. Accuracy of administrative code data for the surveillance of healthcare-associated infections: A systematic review and meta-analysis. Clin Infect Dis 2014;58:688-96. [ Links ]
33. Trybou J, Spaepen E, Vermeulen B, Porrez L, Annemans L. Hospital-acquired infections in Belgian acute-care hospitals: Financial burden of disease and potential cost savings. Acta Clin Belg 2013;68:199-205. [ Links ]
34. Márquez Cid M, Valera Niñirola I, Chirlaque López MD, Tortosa Martínez J, Párraga Sánchez E, Navarro Sánchez C. Validación de los códigos diagnósticos de cáncer de colon y recto del conjunto mínimo básico de datos. Gaceta Sanitaria 2006;20:266-72. [ Links ]
35. López Izquierdo R, Asensio Villahoz P, Vicente Vírseda JA, González Manzano I, Udaondo Cascante MA. Tuberculosis pathology attended in emergency through the analysis of the hospital discharges MBDS in the West Valladolid Area, Spain (2002-2006). Revista Española de Salud Pública 2009;83:279-90. [ Links ]
36. Merino Peralta A. Validez del conjunto mínimo básico de datos (CMBD) como fuente de información para detectar factores de riesgo y efectos adversos postoperatorios (Internet). (Acceso 23 noviembre 2012). Disponible en: http://academica-e.unavarra.es/handle/2454/6106. [ Links ]
37. Ramalle-Gomara E, Ruiz E, Serrano M, Bartulos M, Gonzalez MA, Matute B. Validity of discharge diagnoses in the surveillance of stroke. Neuroepid 2013;41:185-8. [ Links ]
38. Ribera A, Marsal JR, Ferreira-González I, Cascant P, Pons JMV, Mitjavila F, et al. Predicting in-hospital mortality with coronary bypass surgery using hospital discharge data: Comparison with a prospective observational study. Rev Esp Cardiol 2008;61:843-52. [ Links ]
39. Pérez Schael I. Aplicación universal de la vacuna de rotavirus: impacto en la mortalidad y hospitalizaciones por diarrea. Revista de la Sociedad Venezolana de Microbiología 2011;31:104-11. [ Links ]
40. Koch J, Wiese-Posselt M, Remschmidt C, Wichmann O, Bertelsmann H, Garbe E, et al. Background paper to the recommendation for routine rotavirus vaccination of infants in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:957-84. [ Links ]
41. Waisbourd-Zinman O, Ben-Ziony S, Solter E, Chodick G, Ashkenazi S, Livni G. The percentage of nosocomial-related out of total hospitalizations for rotavirus gastroenteritis and its association with hand hygiene compliance. Am J Infect Control 2011;39:166-8. [ Links ]
![]() Correspondence:
Correspondence:
Olga Redondo González
Research Support Unit
Hospital General La Mancha Centro
Avda. Constitución, 3
13600 Alcázar de San Juan, Ciudad Real. Spain
e-mail: oredgon@gmail.com
Received: 30-09-2014
Accepted: 17-11-2014











 text in
text in