My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.4 Madrid Apr. 2015
ORIGINAL PAPERS
Fecal calprotectin as a biomarker of inflammatory lesions of the small bowel seen by videocapsule endoscopy
Calprotectina fecal como predictor de lesiones inflamatorias en intestino delgado diagnosticadas con cápsula endoscópica
Juan Egea-Valenzuela, Fernando Alberca-de-las-Parras and Fernando Carballo-Álvarez
Unit of Gastrointestinal Endoscopy. Service of Digestive Diseases. Hospital Clínico Universitario "Virgen de la Arrixaca". Murcia, Spain
ABSTRACT
Introduction: The levels of calprotectin in the stools are proportional to neutrophil activity in the enteric lumen, so fecal calprotectin is a useful intestinal inflammatory biomarker. It is an extended tool as predictor of colonic pathology but there is scare evidence about its utility in the small bowel.
Objective: To test the yield of fecal calprotectin to detect lesions in the small bowel.
Material and methods: We have retrospectively included 71 patients sent for small bowel capsule endoscopy in study for suspected inflammatory bowel disease. All of them had a determination of fecal calprotectin and had been sent to colonoscopy with no findings. Patients have been divided in groups: A, fecal calprotectin < 50 µg/g; B, fecal calprotectin: 50-100 µg/g; C, fecal calprotectin > 100 µg/g, and we have analyzed which of them presented inflammatory lesions in capsule endoscopy studies.
Results: The rate of patients with signi ficative lesions was 1 out of 10 (10%) in group A, 6 out of 24 (25%) in group B, and 21 out of 34 (62%) in group C. If we consider levels over 50 µg/g pathologic, fecal calprotectin presents sensitivity: 96%, specificity: 23%, NPV: 90% and PPV: 56%. If we consider levels over 100 µg/g pathologic these values are sensitivity: 75%, specificity: 67%, NPV: 79% and PPV: 62%.
Conclusions: Fecal calprotectin has high sensitivity but not so good specificity for predicting small bowel lesions after a normal colonoscopy. In daily practice it will be more useful to establish in 100 µg/g the limit to indicate capsule endoscopy studies.
Key words: Chron's disease. Capsule endoscopy. Calprotectin.
RESUMEN
Introducción: los niveles de calprotectina en heces son proporcionales a la actividad neutrofílica en la luz intestinal, convirtiéndola en un marcador inflamatorio enteral útil. Se ha extendido su uso como predictor de patología colónica, pero hay escasa evidencia en cuanto a su utilidad en lesiones de intestino delgado.
Objetivo: comprobar la capacidad de la calprotectina fecal para detectar lesiones en intestino delgado.
Material y métodos: se ha recogido datos retrospectivamente de 71 pacientes sometidos a estudios de cápsula de intestino delgado por sospecha de enfermedad inflamatoria. Todos se habían sometido a una determinación de calprotectina fecal y a una colonoscopia normal. Hemos estratificado en grupos: A, CPF < 50 µg/g; B, CPF: 50-100 µg/g; C, CPF > 100 µg/g, analizando en la cápsula las lesiones sugestivas de enfermedad inflamatoria.
Resultados: la proporción de pacientes con lesiones significativas fue 1 de 10 (10%) en el grupo A, 6 de 24 (25%) en el grupo B, y 21 de 34 (62%) en el grupo C. Tomando un valor mayor de 50 µg/g como patológico, la calprotectina fecal tiene una S: 96%, E: 23%, VPN: 90% y VPP: 56%. Tomando 100 µg/g como referencia estos valores son S: 75%, E: 67%, VPN: 79% y VPP: 62%.
Conclusiones: la calprotectina fecal es sensible pero inespecífica para predecir qué pacientes presentarán lesiones en intestino delgado tras una colonoscopia normal. En la práctica clínica es más útil establecer en 100 µg/g el límite para indicar un estudio de cápsula endoscópica.
Palabras clave: Enfermedad de Crohn. Cápsula endoscópica. Calprotectina.
Introduction
Calprotectin is a calcium and zinc binding protein present in the whole organism but most frequent in neutrophils and monocytes, in which it represents 5% of all their protein content and 60% of their cytosol. Calprotectin has bactericidal and fungicidal activity and its plasmatic levels rise significantly in cases of infectious and inflammatory processes. It is also present in the stools, and fecal calprotectin (FC) levels are higher than those in blood (1).
Levels of calprotectin can be measured in many biologic fluids, tissue specimens, stools... representing a potentially relevant marker of neutrophil activity. FC has been proposed as a biomarker of enteric inflammation and neoplastic lesions as its presence in the stools is directly proportional to neutrophil activity in the intestinal lumen (1,2).
There is consistent evidence about usefulness of FC in colonic pathology, being considered as a parameter that can determinate whether a symptomatic patient will need a lower endoscopy or not. Many authors have proposed that FC can be useful to distinguish between organic and functional pathology and to choose those patients who are more likely to benefit from performance of a colonoscopy (3-5). However, evidence about levels of FC and pathology of the small bowel is scarce and sometimes contradictory, so these recommendations cannot be extended to endoscopic studies of these portions of the gastrointestinal tract. This is why we have decided to analyze our experience to test the capacity of FC to predict pathologic small bowel capsule endoscopy studies.
Methods
We have selected from our capsule endoscopy (CE) database those patients in which a small bowel study has been performed because of abdominal pain, chronic diarrhea or suspected inflammatory bowel disease (IBD), and who had a measurement of FC as part of their diagnostic study. In our center levels of FC are determinate by automated enzyme immunoassay analyzer (estimated sensitivity of 95%). Levels under 50 µg/g are considered normal, and levels between 50-100 µg/g of uncertain clinic significance. In all these patients lower conventional endoscopic studies had been carried out with no findings, and we excluded those individuals who had been taking non steroidal anti inflammatory drugs in the six weeks previous to CE.
Seventy-one patients fix the inclusion criteria (22% of our entire database) along 21 months. There are 46 women (65%) and 25 men (35%), with a mean age of 46 years old (16-78). In all the patients (100%) a colonoscopy had been carried out without findings; only in 19 patients (26%) endoscopists also performed ileoscopy, being normal; in 18 individuals (25%) a gastroscopy had been also performed.
In 7 patients we suspected strictures of the small bowel due to their symptoms or altered radiologic tests. All of them underwent a patency capsule test with no excretion of this in 3 patients. In these cases, CE was contraindicated and is not included in this study. In the other 4 patients with normal excretion of the patency capsule CE was performed without complications.
The mean time between determinations of FC and performance of CE studies was 81 days, and in 16 patients there were more than one FC determination. In these cases we have used the one closest to CE.
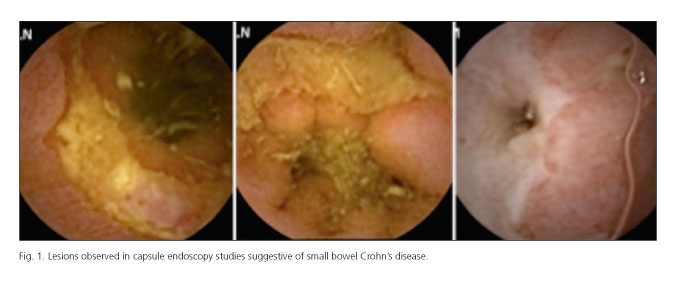
CE studies have been read and informed by two experienced endoscopists. These had access to clinic data of the patients when reading the studies, including the results of previous endoscopic procedures, laboratory tests and FC levels. Diagnosis of Crohn's disease (CD) of the small bowel was based on the findings of villous edema and erythema, mucosal denudation, aphtha or ulcers and stenosis (Fig. 1). In those cases in which lesions have been observed in CE, such as we use to do in our daily practice in our Unit, the Lewis score (LS) has been used to assess the inflammatory activity as recommended by Gralnek et al.: LS < 135, normal or insignificant; LS: 135-790, mild; LS > 790, severe mucosal inflammation (6).
Referring to findings in CE studies these have been classified in three groups: Normal studies, studies with non-significative findings and studies with significative lesions and high probability for IBD. In the group of CE with non-significative findings we have included small lesions, in most of the cases mild mucosal erosion of denudation, limited areas with mild oedema or erythema, lymphangiectasia or vascular lesions... In conclusion lesions not related to IBD or insufficient for a diagnosis of small bowel CD. When performing statistical analysis normal and non-significative studies have been included together.
Results
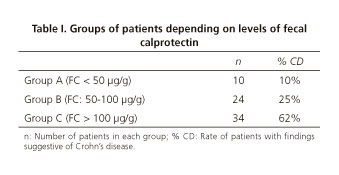
The 68 patients in which CE was performed have been stratified in the basis of their FC levels. FC was lower than 50 µg/g in 10 cases (group A); in 24 patients levels of FC were among 50-100 µg/g (group B); in the other 34 individual levels of FC were higher than 100 µg/g (group C). These data are summarized in table I.
In group A, only 1 out the 10 patients presented findings suggestive of CD in his CE study. In group B, 6 out of 24 patients (25%) presented significative lesions. In this group one patient (a middle-aged woman) who had received radiotherapy for a gynecologic neoplasm was not diagnosed of CD but severe radiation enteritis was observed. Among the other 18 patients in group B, 12 presented with normal studies and 6 with non-significative lesions. In group C, 21 studies out of 34 (62%) were compatible with CD. Among the rest 8 studies were considered normal and in 5 cases non-significative lesions were observed. It is to remark that in this group most of the patients (26 out of 34) had levels of FC between 100-200 µg/g. From those 8 individuals with FC > 200 µg/g, 6 presented significative lesions (75%). In these 2 patients with high FC and no diagnosis of CD it is striking that one of them presented continuously levels of FC over 500 µg/g, but after a second lower endoscopy with biopsies and several radiologic studies no diagnosis has been achieved yet.
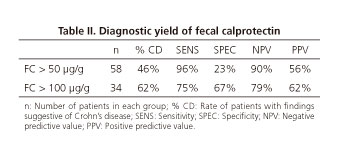
If we consider levels of FC under 50 µg/g as normal, there are 58 patients with pathologic levels of FC in our series. Only 27 of these (46%) have significative lesions in CE, and 31 have normal or non-significative studies (20 and 11 patients respectively). We also have 10 patients with normal FC and 1 of them (10%) had positive findings for CD. This means that FC has Sensitivity: 96%, specificity: 23%, negative predictive value (NPV): 90% and positive predictive value (PPV): 56% (Table II).
If we consider levels of FC under 100 µg/g as normal, there are 34 patients with pathologic levels in our series. Twenty-one of these (62%) were diagnosed of small bowel CD, and 13 studies were considered normal or non-significative (8 and 5). Among the 34 patients with normal FC, only 7 (20%) presented significative lesions in CE, and 27 (79%) had normal or non-significative studies (21 and 6). In this case we obtain sensitivity: 75%, specificity: 67%, NPV: 79% and PPV: 62% (Table II).
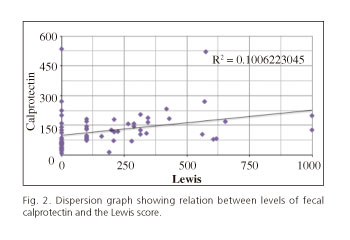
In the daily practice of our endoscopy unit we use the Lewis score (LS) to assess the significance and severity of the lesions observed in CE. In figure 2 we represent the relation between the levels of FC and LS in each of the studies as a dispersion graph.
As it has been already said in all the patients included in our study a lower endoscopy had been performed before CE. Although these were patients with suspected CD only in 19 cases ileoscopy has been reported (normal in all the cases). Eleven of these patients are included in group B, and only 2 of them presented significative lesions in CE. The other 8 patients were included in group C, and 5 of them were diagnosed of CD. In 52 patients ileoscopy was not performed (or at least it was not reported). Among these 52 were the 3 patients with no excretion of the patency capsule in which CE was contraindicated. In the remaining 49 patients: 10 were included in group A, with only one diagnosis of CD; 13 individuals in group B, with 4 CE studies with significative lesions; and 26 in group C, with 16 CE studies compatible with CD. In conclusion, 7 out of 19 (37%) patients with normal ileoscopy presented significative lesions in CE; 21 out of 49 (42%) patients without ileoscopy were diagnosed of small bowel CD in CE. There is not significative difference between those patients with normal ileoscopy and those in which the affectation of terminal ileum is unknown in terms of risk for presenting small bowel inflammatory lesions.
In some CE studies we observed ulcerated stenosis, but in most of the cases the capsule run trough them with no complications. Nevertheless, retention of the capsule has been registered in two patients with small bowel strictures. These patients had not referred any symptoms in relation with stenosis and the test with patency capsule had not been performed in any of these cases. Both patients presented with multiple and complex stenosis and were sent for elective surgical treatment. Capsule retention did not worsen the clinical situation of these patients and did not determine the time for surgery.
Discussion
FC has become a very common analytic parameter for general gastroenterologists and those with higher dedication to IBD. Its use has extended for individuals with suspected IBD, and in the follow-up of confirmed IBD patients. In the first case FC has been proposed as a biomarker with capacity to discriminate in which patients there is higher probability to find inflammatory lesions in a lower endoscopy. This way FC could assess if a patient is more likely to present a functional gastrointestinal disorder or IBD and determinate who will benefit the most from a colonoscopy (3-5,7). The use of FC is also accepted in the pediatric age but some authors indicate that its precision is higher in adults (7-9).
Although not very extended, the use of FC has also been proposed as a fecal marker in colorectal cancer screening, especially in first degree relatives (10). However, FC is very efficient in predicting the presence of advanced neoplasia but not adenomas, so it is not recommended for screening in general population (11).
FC has also shown great utility in the follow-up of patients with IBD, because it can predict mucosal healing and the response to treatments as its levels seem to be related to inflammatory activity in the colon and can be related to endoscopic findings too, as some authors have reported (12,13).
In those patients with suspected IBD and normal colonoscopy, when FC is high it seems natural to search for small bowel inflammatory lesions. In daily practice it has become very usual to perform small bowel CE in these cases, although evidence relating FC levels and small bowel pathology is scarce and sometimes contradictory. In one series of patients who underwent barium small bowel X-ray examinations due to suspected IBD authors indicate that FC is better than C-reactive protein and erythrocyte sedimentation rate for predicting which patients are more likely to present inflammatory lesions (14). Referring to endoscopic studies there are only a few case series in literature relating levels of FC to findings in small bowel CE. These studies are different and difficult to compare in terms of methodology and design and their results are disparate. In one of them the authors conclude that certain fecal markers, FC among them, are useful to predict colonic lesions but not small bowel pathology (15). On the other hand, some other case series report that FC can be useful to decide which patients need small bowel endoscopy. In one of these series authors inform that FC is a useful tool to decide which patients will benefit from small bowel CE after normal bidirectional endoscopy (16). In addition, in this study it is remarkable that FC is especially significative when levels are high, suggesting that CE must not be performed if FC < 100 µg/g and that the greatest diagnostic yield is obtained when FC > 200 µg/g. One last study relates levels of FC and C-reactive protein to findings in CE in 30 patients with CD (17). The conclusions of this study are that FC and other inflammation biomarkers correlate well with inflammatory lesions seen in CE, and in the case of FC this correlation is positive in the moment of diagnosis and in the follow-up.
The results of our series are congruent with these last two referred studies (16,17). Our conclusions are that FC has very good sensitivity and negative predictive value, but low specificity and positive predictive value when normal levels are set at 50 µg/g (as it is the case of most laboratories). If we consider FC < 100 µg/g as normal, sensitivity and negative predictive value descend (but are still good) and specificity and positive predictive value improve significantly. From a practical point of view and bringing this results to daily activity in our endoscopy units, we understand that cost-effectiveness of small bowel CE in suspected CD after normal colonoscopy is higher when FC > 100 µg/g. In patients with FC: 50-100 µg/g, the indication of small bowel studies should be supported by high clinical suspicion and additional altered laboratory tests as cost-effectiveness of FC is lower in this group. In general, in patients with FC < 50 µg/g small bowel CE should not be performed.
Disposable evidence correlating FC with small bowel CD is scarce. Is will be necessary to perform more studies, prospective if possible, in this field and try to correlate FC with other inflammatory markers too, with the aim of defining more closely those factors associated to higher cost-effectiveness when indicating CE studies in patients with suspected small bowel CD.
References
1. Rodrigo L. Calprotectina Fecal. Rev Esp Enferm Dig 2007;99:683-8. [ Links ]
2. Summerton CB, Longlands MG, Wiener K, et al. Faecal Calprotectin: A marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol 2002;12:841-5. [ Links ]
3. Bonnin Tomas A, Vila Vidal M, Rosell Camps A. Fecal calprotectin as a biomarker to distinguish between organic and functional gastrointestinal disease. Rev Esp Enferm Dig 2007;99:689-93. [ Links ]
4. García-Sánchez MV, González R, Iglesias-Flores E, et al. Precisión diagnóstica de la calprotectina fecal para predecir una colonoscopia patológica. Med Clin (Barc) 2006;127:41-6. [ Links ]
5. Schoepfer AM, Trummler M, Seeholzer P, et al. Discriminating IBD from IBS: Comparison of the test performance of fecal markers, blood leukocytes, CRP and IBD antibodies. Inflam Bowel Dis 2008;14:32-9. [ Links ]
6. Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy scoring index for small bowel mucosa inflammatory change. Aliment Pharmacol Ther 2008;27:146-54. [ Links ]
7. Rodriguez-Moranta F, Lobaton T, Rodriguez-Alonso L, et al. Calprotectina fecal en el diagnóstico de enfermedades inflamatorias. Gastroenterol Hepatol 2013;36:400-6. [ Links ]
8. van Rheenen PF, Van de Vijver E, Filder V. Fecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010;341:c3369. [ Links ]
9. Bunn SK, Bisset WM, Main MJ, et al. Fecal calprotectin: Validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2001; 33:14-22. [ Links ]
10. Kristinsson J, Nygaard K, Aadland E, et al. Screening for first degree relatives of patients operated for colorectal cancer: Evaluation of fecal calprotectin vs. Hemoccult II. Digestion 2001;64:104-10. [ Links ]
11. von Roon AC, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol 2007;102:803-13. [ Links ]
12. Roseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: A predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol 2004;39:1017-20. [ Links ]
13. Sipponen T, Savilahti E, Kolho KL, et al. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis 2008;14:40-6. [ Links ]
14. Dolwani S, Metzner M, Wassell JJ, et al. Diagnostic accuracy of faecal calprotectin in prediction of abnormal small bowel radiology. Aliment Pharmacol Ther 2004;20:615-21. [ Links ]
15. Sipponen T, Haapamaki J, Savilahti E, et al. Fecal calprotectin and S100A12 have low utility in prediction of small bowel Crohn's disease detected by wireless capsule endoscopy. Scand J Gastroenterol 2012;47:778-84. [ Links ]
16. Koulaouzidis A, Douglas S, Rogers MA, et al. Fecal calprotectin: A selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scan J Gastroenterol 2011;46:561-6. [ Links ]
17. Höög CM, Bark LÅ, Broström O, et al. Capsule endoscopic findings correlate with fecal calprotectin and C-reactive protein in patients with suspected small bowel Crohn's disease. Scand J Gastroenterol 2014;49:1084-90. [ Links ]
![]() Correspondence:
Correspondence:
Juan Egea-Valenzuela.
Unit of Gastrointestinal Endoscopy.
Service of Digestive Disease.
Hospital Clínico Universitario "Virgen de la Arrixaca".
Ctra. Madrid-Cartagena, s/n.
30120 El Palmar, Murcia
e-mail:
juanegeavalenzuela@gmail.com
Received: 12-10-2014
Accepted: 29-01-2015











 text in
text in