Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.4 Madrid abr. 2015
LETTERS TO THE EDITOR
Acute liver failure in a patient consuming Herbalife products and Noni juice
Fallo hepático agudo en paciente consumidora de productos de Herbalife y zumo de Noni
Key words: Acute liver failure. Liver toxicity. Noni. Herbalife.
Palabras clave: Fallo hepático. Toxicidad hepática. Noni. Herbalife.
Dear Editor,
There is a perception in the general population that herbal products are "natural" and safe without side effects.
Case report
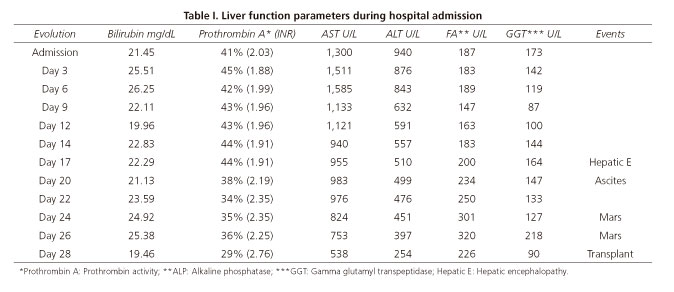
We present a case of a 56-year-old female patient without previous liver disease. No relevant medical history was reported with only regular consumption of Herbalife products and Noni juice. She was admitted to the emergency room with diffuse nonspecific abdominal pain, nausea, and mucocutaneous jaundice. Physical examination showed a conscious and oriented patient with no neurological impairment. Routine laboratory tests were performed at the time of admission showing: ALT 940 U/L, AST 1300 U/L, GGT 173U/L, ALP 187 U/L, LDH 431 U/L, total bilirubin 21.45 mg/dL, and a prothrombin activity of 41% (INR 2.03).
A more detailed study was performed including viral serology: HAV, HBV, HCV, HEV, CMV, EB, VZV and HHV 6-8 with negative IgG and IgM markers. HIV serology was also negative. HCV RNA and HBV DNA in blood were negative. Other tests including Coxiella, T. cruzi, Treponema and Brucella showed negative results for IgG and IgM. Wilson disease, alpha 1 antitrypsin deficiency and autoimmune hepatitis were ruled out.
Imaging tests, including abdominal ultrasound and magnetic resonance cholangiopancreatography, discarded biliopancreatic pathology. The evolution of the patient was torpid and laboratory parameters did not improve as shown in table I.
A transjugular liver biopsy was made and the histologic findings were suggestive of lobular hepatitis with confluent necrosis (subfulminant hepatitis) and nodular regeneration.
During the third week of admission, the patient suffered an episode of grade II/IV hepatic encephalopathy (West Haven classification) and showed clinical worsening with development of ascites. The patient progressed to acute liver failure and liver transplant was performed with good outcome.
Discussion
Between 2 and 10% of the reported cases of liver failure in Spain have been attributed to herbal products or dietary supplements. These data should be interpreted with caution as they are described in the section of possible causes not associated with regular drugs (1,2). In the case described above, once the main etiologies of acute hepatitis were ruled out, the symptoms were attributed to the consumption of Noni (Morinda Citrifolia) and a wide range of products registered under the trademark of Herbalife. If we apply the RUCAM index for acute liver damage in the case described we obtain a consistent score with a degree of "probable" (3). It is difficult to determine whether the etiology of the liver failure was only related to the use of one of these substances or its combination.
So far, there are seven cases described in the literature of liver toxicity related to Noni (4,5), with one of them presenting as an acute liver failure that required liver transplantation (4). The reported cases of hepatotoxicity secondary to Herbalife products are more abundant in the literature and several cases of liver failure requiring liver transplant have been described (6,7). In Spain, Manso et al. published a series of 21 cases (8).
In the case of Noni, the toxicity is attributed to the anthraquinones. They produce oxygen free radicals which result in oxidative stress due to the depletion of intracellular reduced glutathione as well as an altered mitochondrial membrane potential. This ends up producing lipid peroxidation and finally cell death (9).
Toxicity induced by Herbalife products could be related to several components. Due to the large number of different products offered by Herbalife, some of them unlabelled and not showing the exact components, it is very difficult to determine the specific agent that may cause liver damage. We consider that the liver damage is caused by a mix of substances acting by their own or interacting with each other (8).
We believe that a greater control by health authorities would be necessary in the production and distribution of herbal products. It is also important to establish the safety and efficacy of these products in order to warn the citizens of their potential adverse effects.
Francisco Garrido-Gallego, Raquel Muñoz-Gómez,
Carolina Muñoz-Codoceo, Pilar Delgado-Álvarez,
Inmaculada Fernández-Vázquez
and Gregorio Castellano-Tortajada
Department of Gastroenterology. Hospital Universitario
12 Octubre. Madrid, Spain
References
1. Andrade RJ, Lucena MI, Fernández MC, et al. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005;129:512-21. [ Links ]
2. Ibañez L, Perez E, Vidal X, et al. Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatol 2002;37:592-600. [ Links ]
3. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323-30. [ Links ]
4. Stadlbauer V, Weiss S, Payer F. Herbal does not all mean innocuous: The sixth case of hepatotoxicity associated with Morinda citrofolia (noni). Am J Gastroenterol 2008;103:2406-7. [ Links ]
5. Yu EL, Sivagnanam M, Ellis L, et al. Acute hepatotoxicity after ingestion of Morinda citrifolia (Noni Berry) Juice in a 14-year-old Boy. J Pediatr Gastroenterol Nutr 2011;52:222-4. [ Links ]
6. Schoepfer AM, Engel A, Fattinger K, et al. Herbal does not mean innocuous: Ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife products. J Hepatol 2007;47:521-6. [ Links ]
7. Elinav E, Pinsker G, Safadi R, et al. Association between consumption of Herbalife nutritional supplements and acute hepatotoxicity. J Hepatol 2007;47:514-20. [ Links ]
8. Manso G, López-Rivas L, Salgueiro ME, et al. Continuos reporting of new cases in Spain supports the relationship between Herbalife products and liver injury. Pharmacoepidemiol Drug Saf 2011;20:1080-7. [ Links ]
9. Wang MY, West BJ, Jensen CJ, et al. Morinda citrifolia (Noni): A literature review and recent advances in Noni research. Acta Pharmacol Sin 2002;23:1127-41. [ Links ]











 texto en
texto en