Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.5 Madrid may. 2015
REVIEW
Groove pancreatitis
Pancreatitis del surco
Anna Pallisera-Lloveras1, José Manuel Ramia-Ángel2, Carles Vicens-Arbona1 and Andrés Cifuentes-Rodenas1
1Hospital Son Llàtzer. Palma de Mallorca, Spain.
2Hospital Universitario de Guadalajara. Guadalajara, Spain
ABSTRACT
Groove pancreatitis is a type of chronic pancreatitis that affects the area between the pancreatic head, the duodenum and the common bile duct and can simulate, mask or coexist with pancreatic carcinoma. It should be considered in the differential diagnosis of pancreatic masses or duodenal stenosis. It is a rare disease but is probably underdiagnosed. Several names are used to refer to it in the literature, a fact that makes it difficult to extract precise information. Here we present an exhaustive review of the relevant literature on the entity and discuss its clinical features, diagnosis and therapy.
Key words: Groove pancreatitis. Computed tomography. MRI. Surgery. Revision.
RESUMEN
La pancreatitis del surco o groove pancreatitis es un subtipo muy infrecuente de pancreatitis crónica de etiología incierta que ocurre en el surco pancreático duodenal, y suele afectar a varones alcohólicos de mediana edad. Debido a su baja incidencia probablemente haya sido en el pasado infradiagnosticada. El diagnóstico diferencial con otras entidades duodenales y pancreáticas primordialmente el cáncer de páncreas, pese a los avances diagnósticos es aún muy difícil. No existen guías terapéuticas definidas que nos permiten el tratamiento más óptimo.
Hemos efectuado una revisión exhaustiva de la literatura de la clínica, patogenia, histología,métodos diagnósticos, diagnóstico diferencial y maniobras terapéuticas de la pancreatitis del surco que permiten un mejor conocimiento de esta entidad.
Palabras clave: Pancreatitis. Tomografía computarizada. Imagen por resonancia magnética. Cirugía. Revisión.
Introduction
The pancreatic duodenal groove is a small area confined between the pancreatic head, duodenum and bile duct (1). The term groove pancreatitis (GP) refers to a type of chronic pancreatitis that primarily affects this area of the pancreas, while the rest of the organ remains largely intact (2-5). It was first described, in 1973, by Becker, using the German term "Rinnenpankreatitis" (6); in 1982, Solte et al. (2) translated it as "groove pancreatitis". It is a rare but probably underdiagnosed disease (7).
In 1991 Becker and Mischke (3) described two forms of GP: Pure and segmental. In the pure form, scar tissue affects only the pancreatic groove; the parenchyma and main pancreatic duct (MPD) remain preserved (2-5,8,9). In the segmental form, the scar tissue extends to the dorso-cranial portion of the pancreatic head, near the duodenal wall, and the MPD presents stenosis (2-5,8,9). However, the distinction between the two forms is not always clear-cut (10).
A variety of terms have been used to refer to GP in the literature: For example, cystic dystrophy of heterotopic pancreas or duodenal dystrophy, first reported, in 1970, by the French authors Potet and Duclert (11). Other names include duodenal/paraduodenal wall cyst, pancreatic hamartoma of the duodenal wall, myoadenomatosis, Brunner's gland hamartoma and paraduodenal pancreatitis have also been used to describe this entity. These differences in the terminology used make it difficult to extract precise information (4,9,12-17).
GP is more common in men, tending to appear in the fourth or fifth decade of life, with a history of severe chronic alcoholism. Some series also associate heavy smoking (9,15,16,18-20). GP has also been described in women (9,21,22) in patients without a history of alcoholism (9,17,21,22) and in younger patients, although the incidence is significantly lower (10,21).
The importance of GP is that it can mimic pancreatic carcinoma (PC) (9,18,23-28): It may coexist with PC or even mask it (29). GP should be considered in the differential diagnosis of pancreatic masses or duodenal stenosis (30-32).
For these reasons we conducted a thorough review of the literature on GP focusing on clinical, diagnostic and therapeutic issues. We performed a MEDLINE search from 1966 to 2014 looking for the terms "groove pancreatitis", "paraduodenal pancreatitis", "paraduodenal wall cysts", and "duodenal cystic dystrophy". Only articles in English, Spanish or French were searched. Finally, 68 articles were included in this review; of these, most were clinical case studies with fewer than four cases each (5-7,19,22,23,25,28-54). The table I summarizes the most relevant series described (2,3,9,11,15-18,20,21,55-60).
Clinical presentation
The most common clinical presentation of GP is pain in the upper abdomen, associated with recurrent postprandial vomiting and weight loss, primarily due to duodenal obstruction (15-17,20). Sometimes it may be associated with diarrhea (17,40) or diabetes mellitus (17) and less frequently to jaundice; when present, jaundice is usually fluctuating, unlike the progressive jaundice secondary to PC (14,17,21,61). The course of GP is usually chronic, with the duration of clinical symptoms ranging from weeks to more than a year (42). Certain complications of GP have been described, such as perforation and gastrointestinal bleeding and rarely malignant degeneration of heterotopic pancreas (15,21,8).
Pathogenesis
The real pathogenesis of GP has not been clearly identified (8,30,42,43). Almost all authors agree that alcohol is a predisposing factor and the main cause of the disease, in all its clinical manifestations (2,4,8,9,12,14,40-42).
One of the most frequently reported mechanisms is altered pancreatic secretion through Santorini's duct (SD) related to aggression caused by alcohol (2,4,8,12,14,18, 43,62). When it is disturbed, the pancreatic secretion via the SD is directed towards the body of the pancreas, to Wirsung's duct, which forms an acute angle, causing interference with the flow and an accumulation of temporal secretion at the top of the pancreatic head (30). The increased intraductal pressure in the SD facilitates the formation of pseudocysts and leakage of pancreatic juice into the groove (29,34). This alteration can be caused by anatomical causes in the minor papilla (4,5,14,21,29,43) or by incorrect papillary function (4,5,21,33,34):
1. Anatomical causes: Tumors occluding the minor papilla and SD (30,40), a closed SD (12), pancreas divisum (4,5,12,14) and heterotopic pancreas in the duodenal wall may reflect incomplete involution of the dorsal pancreas in this area and contribute to obstruction of the flow (4,14,18,35). This heterotopic pancreatic tissue, under the stimulation of tobacco and alcohol, can cause recurrent episodes of painful "ischemic" pancreatitis (29).
2. Functional causes: Hyperplasia of Brunner's glands (5,8,18,21,29,40) and excessive chronic alcohol and/or tobacco can cause dysfunction or occlusion of the minor papilla (5,29). Chronic alcohol consumption increases the intraductal protein concentration which in turn increases the viscosity of the pancreatic juice, exacerbating the inflammatory process and ductal obstruction (5,8,18,30,41,42).
Peptic ulcers (2,4,5,18,40,42,43,62), both gastric and duodenal, have been postulated as potential triggers of GP, associated with the segmental form in 41% of cases in Solte et al.'s series (2). Other factors include gastric hypersecretion (4,40), gastric resections (2,4,5,18,37,40,42,43,62), true cysts of the duodenal wall (2,4,18,42,43,62), cysts of the pancreatic head (36,42) and previous biliary system illness (2,5,40,42). Recently a case of GP caused by isoniazid was described, possibly via toxic or autoimmune-mediated effects (53).
Histology
The different names the entity has received in the literature are due to the variations in its macroscopic and microscopic appearance.
Macroscopic appearance
Gross examination of the duodenal wall, especially in the vicinity of the minor papilla, is very important for diagnosis of GP (12). In most cases some degree of thickening and scarring of the duodenal wall is observed with trabeculae that are often associated with cysts (5,9,12,14,15,18,26,39,63). These cysts, located in the submucosa, may contain clear liquid, white granular material or stones, and may extend to the area of the groove, compressing the bile duct (5,12,14,26).
At macroscopic level there are two subtypes of GP: a) The "cystic" type, characterized by multiple cysts protruding from the mucosa of the suprampullary duodenum, ranging in size between 1 and 10 cm (26); they form a "paraduodenal wall cyst" or simulate intestinal duplication (12); b) the "solid" type, with cysts smaller than 1 cm in diameter but characterized by a marked thickening of the duodenal wall (26).
The scarring process may also affect the bile duct, which presents a smooth, edematous surface with homogeneous hyalinization (12,26). Other possible findings are thickened folds with ulcerations and retractions in the duodenal mucosa and enlarged lymph nodes in the pancreatic head (5,12,26).
In early stages of GP, the pancreatic head is usually normal or presents edema, mild fibrosis in the dorso-craneal portion with obstruction and dilatation of the SD (26,43,46). As the disease progresses, fibrosis becomes more pronounced and may affect all the pancreatic head (26).
Microscopic appearance
Microscopic examination of the duodenal wall in the vicinity of the minor papilla shows a proliferation of myoid cells wrapping acinar lobules (14,39,63), more prominent in the muscles of the submucosa of the minor papilla and less so in the area of the groove, creating an image that resembles "myoadenomatosis" (4,5,12,16,47) or a pancreatic hamartoma (7,39). The presence of heterotopic pancreatic tissue in the submucosa or muscularis propria of the duodenal wall is frequent (4,15,18,30,43,62).
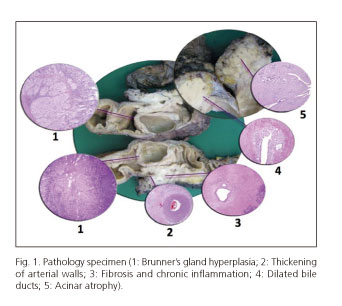
In the myoid cell proliferation, dilated ducts are observed, covered with columnar epithelium (5,7,12,14-16,47), which may erode and become a hypercellular fibroblast-like process, resembling pseudocyst (4,16) and representing the "cystic" variety mentioned above (16). There may be a foreign body reaction with giant cells and accumulation of eosinophils, due to the extravasation of mucoprotein material as these ducts break (7,12,14,16,47,63). A common finding in this entity is Brunner's gland hyperplasia (4,5,12,14-16,26,39,63) which contributes to the thickening of the duodenal wall (12,14,16). Another common feature is neural proliferation, with "hyperplastic" nerves mixing with proliferating islets, creating an image of pseudoinfiltration (12,16) (Fig. 1).
Diagnosis
The preoperative diagnosis of GP is difficult. It requires familiarity with the pathology, review of images by expert radiologists and communication of suspicion of the diagnosis by specialists (22).
Blood test
Pancreatic and liver enzymes may be slightly elevated (4,10,18). Tumor markers are usually normal (4,10).
Abdominal ultrasound (US)
Normally a hypoechoic mass is observed with thickening of the duodenal wall, which causes narrowing of the second duodenal portion and bile duct obstruction (18,43,64).
Wronski et al. (39) postulated that ultrasound findings depend on the stage of the disease and reflect the different pathological processes that occur during the evolution of the GP. For example, in the early stage, when inflammation predominates over fibrosis, a hypoechoic band is evident in the pancreatoduodenal groove corresponding to the inflammatory infiltration, and there may also be a heterogeneous area in the pancreatic head and a moderate thickening of the duodenal wall (39).
In the later stage, when fibrosis is established, hyperechoic thickening of the duodenal wall can be seen resulting from the hypertrophy of submucosa due to hyperplasia of Brunner's glands (39). At this stage a pathognomonic finding is observed: A hyperechoic part of the pancreatic head with anechoic ductal structures, which corresponds to the proliferation of myoma and fibrosis of the adjacent pancreas (39).
Abdominal computed tomography (CT)
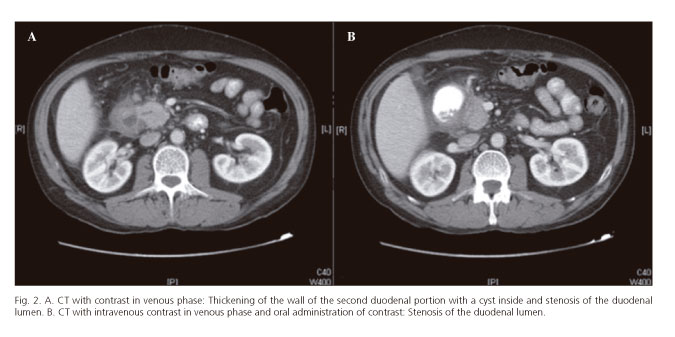
Abdominal CT accurately reflects the histological features of this entity (64). In the pure form, a hypodense laminar mass may be observed between the pancreatic head and duodenum, near the minor papilla (4,8,10,39,52,59,61,62,65), representing scar tissue (64). The delayed contrast uptake observed in some patients is due to the reduced blood flow caused by the proliferation of fibrotic tissue and the constriction of the secondary arteries (4). Cysts of varying size are frequent in the duodenal wall or in the groove (4,8,10,52,59,62,65) (Fig. 2A), or even a multilocular cystic mass (4). The finding of multiple cysts in a thickened duodenal wall with contrast uptake is highly suggestive of cystic dystrophy of heterotopic pancreas (4,15) and duodenal stenosis due to duodenal wall thickening (4,8,59,61) (Fig. 2B). The MPD is usually normal (1,4).
In the segmental form, a hypodense lesion can be seen in the pancreatic head, near the duodenal wall (4,52). The MPD may be slightly dilated toward the body and tail of the pancreas (4,59). The common bile duct may be narrower in the distal part, with mild dilatation of the intra- and extrahepatic biliary tree (4). Peripancreatic vessels are usually preserved, without signs of thrombosis or infiltration, even in cases of extensive disease (1,4,27).
Fibrogastroscopy (FGS)
Fibrogastroscopy may show a swollen polypoid duodenal mucosa with stenosis of the duodenal lumen (7,21,29,31,36,42).
Endoscopic retrograde cholangiopancreatography (ERCP)
ERCP is usually difficult to perform, especially in advanced stages, due to the presence of duodenal stenosis (4,29,41). When it is possible, it may show a smooth distal stenosis of the common bile duct without abnormalities in the MPD or with mild dilatation (4,7,18,36) (Fig. 3). Another possible finding is obstruction or irregular stenosis of SD and its branches, which may contain stones or mucus plugs (4,7,36).
Endoscopic ultrasound (EUS)
According to some authors, EUS may indicate the exact location and extent of the disease, although it is difficult to differentiate between infiltration and inflammation (4,17,29). Thickening and stenosis of the second duodenal portion with intramural cysts are usually observed (15,17,20,52), and smooth stenosis of the common bile duct (21,31,64). In the segmental form, enlargement of the pancreatic head or a heterogeneous hypoechoic mass may be seen infiltrating the duodenal wall (20,41,52,64), with calcifications or pseudocysts and dilatation of the MPD (41,42,64).
Endoscopy-guided FNA biopsy
There are few reports in the literature of the cytologic features of FNA biopsy of GP (44,50). Those published present a great variability depending on the area sampled (4,44,50). If the area sampled has abundant spindle cells (44), large numbers of giant cells (50) or hyperplasia of Brunner's glands, they may mimic neoplasia (44,47,50). This situation is particularly deceptive because the presence of giant cells and hyperplasia of Brunner's glands are among the characteristic features of GP (66). Likewise, an area with fibrosis does not rule out neoplasia, since it is common to find a desmoplastic reaction associated with the adenocarcinoma mimicking abnormal inflammatory changes (4).
Magnetic resonance imaging (MRI)
Some authors consider MRI to be the best diagnostic method for GP, as it allows an evaluation of various pathological aspects of the disease (43,56,58,62,67).
In MRI, GP appears as a laminar mass between the head of the pancreas and duodenum which is hypointense in T1 in comparison with the pancreatic parenchyma (43,55,56,62,64,65). In the T2 sequence it may be hypo-, iso- or slightly hyperintense, according to the duration of the disease. At early subacute stages it is more hyperintense due to edema, whereas in chronic stages it is hypointense due to fibrosis (43,55,56,62,64,65,67). Post-gadolinium images may reveal an irregular enhancement of the mass in the portal phase, but with a progressive, centripetal uptake of contrast at later stages, reflecting the high fibrotic component (43,55,56,64,67).
Cystic lesions may be identified in the groove or in the duodenal wall; they are more obvious and hyperintense in the T2 sequence (20,43,55,56,65,67), and increased thickness or stenosis of the duodenal wall is often seen (4,8,20,43,56,62,65,67). Some authors report that there is some degree of stenosis in the common bile duct, though others observe stenosis in only 50% of patients (63,67). When stenosis occurs it is smooth and tubular or presents a regular pattern of narrowing (8,55,57,65,67). In the segmental form, the pancreatic head or the whole pancreas appear as hypointense in the T1 sequence associated with atrophy of the parenchyma and ductal dilatation, reflecting the progressive loss of glandular cells which are replaced by fibrous tissue (65,67). A thickening of the pancreatic head may also be seen, with progressive narrowing of the MPD (8,43,67). In the pure form, the pancreas is usually hyperintense on the T1 sequence (67).
Magnetic resonance cholangiopancreatography (MRCP)
MRCP is currently the main imaging test used to visualize the MPD and the common bile duct (4). This technique shows the relationship between the ductal system and cystic changes (55,67). It reveals a smooth stenosis of the distal intrapancreatic portion of the common bile duct (4,31,62,64). In the pure form the MPD is normal, but in the segmentary form it usually presents stenosis in the pancreatic head associated with a slight proximal dilatation (4).
Most cases of GP present a widening of the space between the pancreatic ducts, the distal common bile duct and the duodenal lumen, due to a space-occupying lesion in the groove, as well as a marked thickening of the duodenal wall (64,67). The image of "banana-shaped gallbladder" often found in chronic pancreatitis is fairly common in GP (67).
Differential diagnosis
The pancreatic duodenal groove is a small anatomic area where pathological processes affecting the pancreatic head, duodenum, duodenal papilla, the distal common bile duct and retroperitoneum all converge. Therefore, the differential diagnosis of GP includes a spectrum of entities that range from anatomical variants to malignancies (1).
The most important condition to consider in differential diagnosis of GP (principally in the segmental form) is the pancreatic head carcinoma, which in most cases is extremely difficult to distinguish preoperatively (6,10,13,21,23-25,43, 61,64,65,67). CT and MRI are not reliable, especially when the tumor is scirrhous or has a high fibrous component (32,61). Some PC also originate in the groove, making it difficult to distinguish them from the pure form of GP (21,23,29,43,62,63). In 2010, Ishigami et al. (68) reported the utility of the portal venous phase in CT and MRI to distinguish GP from pancreatic groove carcinoma, finding that GP more frequently presents irregular focal enhancement in that phase (68). Recently Kalb et al. (58) achieved a diagnostic accuracy of 87.2% for GP using three strict MRI criteria: focal thickening of the second duodenal portion, an abnormally increased enhancement of the second duodenal portion, and cystic changes in the region of the accessory pancreatic duct. According to the authors, if these three criteria are met, ductal carcinoma of the head of pancreas can be ruled out, with a negative predictive value of 92.9% (58).
The presence of ductal dilatation of the MPD is not useful for differentiating between GP and carcinoma of the head of the pancreas; if the carcinoma affects the MPD the distal portion of duct may present dilatation, and in PC arising in the groove, the MPD is not usually affected (23). Vascular invasion is an important sign for the differentiation of the two entities (4,5,13,27,62,65), especially invasion of the gastroduodenal artery which appears infiltrated in the case of tumor and shifted to the left in the case of GP (1,4,43,61). The presence of cystic lesions in the groove or duodenal wall (normally absent in PC, 1,2,4,59,61,62,64), inflammatory thickening of the duodenal wall (1,2,6,23,30,50,61,62) and stenosis due to scarring of the duodenal lumen all indicate GP (1,2,23,61). Endoscopic biopsy can help, although its usefulness is limited if the cancer is small and does not invade the duodenal mucosa (4). The presence of Brunner's gland hyperplasia is more characteristic of GP and is usually absent in PC (2,6,30).
ERCP and EUS (1,5,6,13,30,42,62,65) may also serve to differentiate these two entities. GP presents a regular smooth bile duct stenosis, while in PC it is irregular and abrupt (1,5,6,13,30,42,62,65,67).
EUS-guided FNA is of great importance in the study of pancreatic lesions because of its high sensitivity and specificity for the diagnosis of PC (44,50). However, its value may be limited depending on the site biopsied (4,44) and its interpretation has to be supported by other complementary tests (44,50).
Other entities which should be included in the differential diagnosis, especially in the pure form, are: Diseases of the common bile duct such as choledochal cyst (21,32) and distal cholangiocarcinoma (4,21,32,42,67), periampullary diseases such as ampulloma (10), duodenal carcinoma (4,10,15,42,43,62,65), periampullary diverticulum (32), ampullary metastasis (49), duodenal hamartoma (21,24), neuroendocrine tumors of the groove (21,32,43,62,65), autoimmune pancreatitis (21,43,62,65) and acute pancreatitis with necrosis or pseudocysts in the groove area (4,32,42,43).
Periampullary carcinomas, including ampulloma and distal cholangiocarcinoma, usually present a hypointense mass in T1 and T2 MRI sequences (43), and MRCP shows an abrupt narrowing of the bile duct rather than the segmentary, long, smooth stenosis found in GP (4,43). The most frequent neuroendocrine tumor in the area of the groove is gastrinoma, which is distinguished from GP by its hyperintensity in T2-weighted images and its hypervascularity on postcontrast images, with peripheral ring-like enhancement on postgadolinum GRE (43). GIST, which present as hypodense lesions in the duodenal submucosa, may mimic GP, although most of them, and most neuroendocrine tumors, present hypervascular lesions (10).
Malignant degeneration of heterotopic pancreas, though rare, deserves special mention because it is almost impossible to differentiate preoperatively from GP. It has been described in some series of surgical specimens of GP (48). In cases of very advanced disease the spread of the tumor has been demonstrated on the posterior aspect of the second duodenal portion, sparing the pancreatic head (48).
Treatment
In a recent study Arvanitakis et al. (20) showed that a stepwise approach to treatment of GP is feasible, effective, and is associated with an acceptable rate of complications.
The treatment of initial acute symptoms with conservative measures (analgesics, pancreatic rest and abstinence from alcohol and tobacco) can be useful in the short term (4,7,13,18,21,2,31,36,37,57,63). Sometimes enteral nutrition is not possible in patients with duodenal stenosis who may require parenteral nutrition (17,20,63).
Somatostatin or its long-acting analogs have been shown to improve the results of the non-surgical approach (20). This may be an alternative to endoscopic treatment, when the papilla cannot be accessed due to compression or the large number of cysts (66), or to surgery in patients with chronic alcohol consumption and an established diagnosis of GP associated with chronic pancreatitis (16,48). Among the drawbacks are the high risk of recurrence of symptoms after treatment cessation (20,48).
Endoscopic treatment, including drainage of the pancreatic duct, dilatation of the stenosis and drainage of cysts, is the mainstay of nonsurgical treatment and can be tailored to the clinical response (20). Endoscopic drainage of SD through the minor papilla (5,18,34,39,63) seems to be feasible only in the early stage of the disease, before the development of severe scarring and duodenal stenosis, which prevents the passage of the endoscope (34,39). Some authors report that the placement of a stent in the minor papilla reduces pain temporarily (57) and can be an alternative to surgery for patients with impaired secretion of pancreatic flow through the minor papilla (4). Endoscopic fenestration via cystoduodenostomy to drain the cysts appears effective in patients with cysts that are few in number and large (13,48). The disadvantages of both these endoscopic techniques are the rate of symptom recurrence and the possible complications due to mobilization or obstruction of the prosthesis (16). Medical and/or endoscopic treatment can be used as first choice in non-severe cases or "bridge" treatments prior to surgery (16). Its results are variable: While in the series of Rebours et al. (17) only 37.5% of patients treated endoscopically showed complete clinical responses in more recent series associating medical and endoscopic treatment complete clinical response rates rise to almost 80% (20).
Thanks to improvements in imaging techniques that can rule out malignancy in some cases of GP, some authors advocate non-surgical procedures to treat this condition (20,66). However, for Patriti et al. (48), that until a system is devised for detecting the degeneration to adenocarcinoma in the heterotopic pancreas, surgery appears to be the safest treatment even when there is no suspicion of malignancy.
Surgery is considered the treatment of choice in GP if symptoms do not improve, when there are complications, or when there is a suspicion of malignancy (5,7,16,18,21,25,31,32,35,42,51). For some authors, the technique of choice is the cephalic pancreaticoduodenectomy, or the Whipple technique (13,16,42,51,60), especially when the main symptom is pain, with or without duodenal obstruction (17).
Pancreatectomy with duodenal preservation has also been performed in some cases and has achieved satisfactory resolution of symptoms (5,42,60), although it is not always possible because of the significant fibrous component of the antral and periduodenal zone (10,16). Another technique which has achieved good results, especially in the case of duodenal involvement, is duodenal resection with preservation of the pancreas (60). A gastroenteroanastomosis may be a solution for patients with marked duodenal stenosis without intractable pain (17) or in patients who are unfit for pancreatectomy.
Duodenopancreatectomy has been shown to be an effective and permanent treatment for the management of GP, controlling the two main symptoms of this condition -pain and weight loss (4,15,18,39,47,48,57,60)- and allowing a complete pathological examination of the surgical specimen in order to confirm diagnosis and rule out malignancy (31,39,48). It has been postulated that the pathogenesis of pain in GP is related to inflammatory mediators, rather than to hyperpressure of the main pancreatic duct, and so techniques of pancreatojejunal derivation, with or without gastric and/or biliary bypass, may provide temporary treatment though symptoms may recur (16,60). Abstinence from alcohol and tobacco, associated with surgery, is critical for good long-term results (16,60).
In a recent study Egorov et al. (60) presented a series of 62 patients, of whom 52 were treated surgically: 29 by pancreaticoduodenectomies, five by pancreatectomy with duodenal preservation, 10 by duodenal resection with preservation of the pancreas and eight by drainage procedures, obtaining symptom control in 85%, 18%, 83% and 18% respectively (60).
Patients with GP undergoing symptomatic treatment or surgical techniques for duodenal preservation require close monitoring due to the risk of a coexisting carcinoma (7,42), especially in the SD (7,30).
Conclusion
GP is a form of chronic pancreatitis which affects the area between the pancreatic head, duodenum and common bile duct which can simulate, mask or coexist with pancreatic carcinoma and should be considered in the differential diagnosis of pancreatic masses or duodenal stenosis. On CT and MRI it appears as a laminar mass between the pancreatic head and duodenum, or in the pancreatic head, with or without dilatation of the MPD. Characteristic features of this entity are inflammatory thickening of the wall and stenosis of the duodenal lumen, and cystic lesions in the mass or in the duodenal wall. Smooth, regular stenosis of the common bile duct and vascular displacement or absence of vascular invasion may also help in the differential diagnosis of this entity, distinguishing it from pancreatic carcinoma. Medical and endoscopic treatment may be helpful initially or in patients who are unfit for surgery, but in some cases, either due to diagnostic uncertainty or due to the need to resolve symptoms that are refractory to medical and endoscopic treatment, pancreatic resection may be necessary.
Acknowledgements
We thank Dr. Mario Serradilla for the pathology image of groove pancreatitis.
References
1. Hernández-Jover D, Pernas JC, González-Ceballos S, et al. Pancreatoduodenal junction: Review of anatomy and pathologic conditions. J Gastrointest Surg 2011;15:1269-81. [ Links ]
2. Solte M, Weib W, Volkholz H, et al. A special form of segmental pancreatitis: "groove pancreatitis". Hepatogastroenterology 1982;29: 198-208. [ Links ]
3. Becker V, Mischke U. Groove pancreatitis. Int J Pancreatol 1991; 10:173-82. [ Links ]
4. Triantopoulou C, Dervenis C, Giannakou N, et al. Groove pancreatitis: A diagnostic Challenge. Eur Radiol 2009;19:1736-43. [ Links ]
5. Tezuka K, Makino T, Hirai I, et al. Groove pancreatitis. Dig Surg 2010;27:149-52. [ Links ]
6. Mohl W, Hero-Gross R, Feifel G, et al. Groove pancreatitis: An important differential diagnosis to malignant stenosis of the duodenum. Dig Dis Sci 2001;46:1034-8. [ Links ]
7. Meesiri S. Groove pancreatitis: Report of one case in Thailand. J Med Assoc Thai 2009;92:1554-9. [ Links ]
8. Kwak SW, Kim S, Lee JW, et al. Evaluation of unusual causes of pancreatitis: role of cross-sectional Imaging. Eur J Radiol 2009;71:296-312. [ Links ]
9. Levenick JM, Gordon SR, Sutton JE, et al. A comprehensive, case-based review of groove pancreatitis. Pancreas 2009;38:e169-75. [ Links ]
10. Raman SP, Salaria SN, Hruban RH, et al. Groove pancreatitis: Spectrum of imaging findings and radiology-pathology correlation. AJR Am J Roentgenol 2013;2013;201:W29-39. [ Links ]
11. Potet N, Duclert N. Cystic dystrophy on aberrant pancreas of the duodenal wall. Arch Fr Mal App Dig 1970;59:223-38. [ Links ]
12. Adsay NV, Zamboni G. Paraduodenal pancreatitis: A clinico-pathologically distinct entity unifying "cystic dystrophy of heterotopic pancreas", "para-duodenal wall cyst" and "groove pancreatitis". Semin Diagn Pathol 2004;21:247-54. [ Links ]
13. Sunnapwar A, Prasad SR, Menias CO, et al. Nonalcoholic, nonbiliary pancreatitis: Cross-sectional Imaging spectrum. AJR Am J Roentgenol 2010;195:67-75. [ Links ]
14. Klöppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol 2007;20 (Supl. 1):S113-31. [ Links ]
15. Rahman SH, Verbeke CS, Gomez D, et al. Pancreatico-duodenectomy for complicated groove pancreatitis. HPB 2007;9:229-34. [ Links ]
16. Casetti L, Bassi C, Salvia R, et al. "Paraduodenal" pancreatitis: Results of surgery on 58 consecutive patients from a single institution. World J Surg 2009;33:2664-9. [ Links ]
17. Rebours V, Lévy P, Vullierme MP, et al. Clinical and morphological features of duodenal cystic dystrophy in heterotopic pancreas. Am J Gastroenterol 2007;102:871-9. [ Links ]
18. Manzelli A, Petrou A, Lazzaro A, et al. Groove pancreatitis. A mini-series report and review of literature. JOP 2011;12:230-3. [ Links ]
19. Latham J, Sanjay P, Watt DG, et al. Groove pancreatitis: A case series and review of the literature. Scott Med J. 2013;58:e28-31. [ Links ]
20. Arvanitakis M, Rigaux J, Toussaint E, et al. Endotherapy for paraduodenal pancreatitis: A large retrospective case series. Endoscopy 2014;46:580-7. [ Links ]
21. Kim JD, Han YS, Choi DL. Characteristic clinical and pathologic features for preoperative diagnosed groove pancreatitis. J Korean Surg Soc 2011;80:342-7. [ Links ]
22. Gupta R, Williams GS, Keough V. Groove pancreatitis: A common condition that is uncommonly diagnosed preoperatively. Can J Gastoenterol Hepatol 2014;28:181-2. [ Links ]
23. Tan CH, Chow PK, Thng CH, et al. Pancreatic adenocarcinoma that mimics groove pancreatitis: Case report of a diagnostic dilemma. Dig Dis Sci 2006;51:1294-6. [ Links ]
24. Adsay NV, Basturk O, Klimstra DS, et al. Pancreatic pseudotumors: Non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol 2004;21:260-7. [ Links ]
25. Varma V, Gandhi V, Bheerappa N, et al. Groove pancreatitis mimicking pancreatic malignancy. Indian J Gastroenterol 2008;27:86. [ Links ]
26. Zamboni G, Capelli P, Scarpa A, et al. Nonneoplastic mimickers of pancreatic neoplasms. Arch Pathol Lab Med 2009;133:439-53. [ Links ]
27. Coakley FV, Hanley-Knutson K, Mongan J, et al. Pancreatic Imaging mimics: Part 1, Imaging mimics of pancreatic adenocarcinoma. AJR Am Roentgenol 2012;199:301-8. [ Links ]
28. Palomeque Jimenez A, Pérez Cabrera B, Navarro Freire F, et al. Groove pancreatitis in the differential diagnosis of pancreatic adenocarcinoma. Cir Esp 2014;92:127-9. [ Links ]
29. Malde DJ, Oliveira-Cunha M, Smith AM. Pancreatic carcinoma masquerading as groove pancreatitis: Case report and review of the literature. JOP 2011;12:598-602. [ Links ]
30. Shudo R, Yazaki Y, Sakurai S, et al. Groove pancreatitis: Report of a case and review of the clinical and radiologic features of groove pancreatitis reported in Japan. Intern Med 2002;41:537-42. [ Links ]
31. Balakrishnan V, Chatni S, Radhakrishnan L, et al. Groove pancreatitis: A case report and review of literature. JOP 2007;8:592-7. [ Links ]
32. Viñolo-Ubiña C, Morales Ruiz J, Heredia Carrasco C, et al. Groove pancreatitis with duodenal stenosis. Rev Esp Enferm Dig 2010; 102:59-60. [ Links ]
33. Shudo R, Obara T, Tanno S, et al. Segmental groove pancreatitis accompanied by protein plugs in Santorini's duct. J Gastroenterol 1998;33:289-94. [ Links ]
34. Isayama H, Kawabe T, Komatsu Y, et al. Successful treatment of groove pancreatitis by endoscopic drainage via the minor papilla. Gastrointest Endosc 2005;61:175-8. [ Links ]
35. Chatelain D, Vibert E, Yzet T, et al. Groove pancreatitis and pancreatic heterotopia in the minor duodenal papilla. Pancreas 2005;30:e92-5. [ Links ]
36. Sanada Y, Yoshida K, Itoh H, et al. Groove pancreatitis associated with true pancreatic cyst. J Hepatobiliary Pancreat Surg 2007;14:401-9. [ Links ]
37. Ito R, Shiba H, Okamoto T, et al. Groove pancreatitis with several cystic lesions around pancreatic head treated conservatively: Report of a case. Case Rep Gastroenterol 2008;2:405-9. [ Links ]
38. Lee TH, Park SH, Lee CK, et al. Ectopic opening of the common bile duct accompanied by groove pancreatitis: Diagnosis with magnetic resonance cholangiopancreatography. Gastrointest Endosc 2010;71:1301-2. [ Links ]
39. Wronski M, Karkocha D, Slodkowski M, et al. Sonographic findings in groove pancreatitis. J Ultrasound Med 2011;30:111-5. [ Links ]
40. Fiscaletti M, Fornelli A, Zanini N, et al. Segmental groove pancreatitis and duodenal gangliocytic paraganglioma with lymph node metastasis: A newly described association. Pancreas 2011;40:1145-7. [ Links ]
41. De Tejada AH, Chennat J, Miller F, et al. Endoscopic and EUS features of groove pancreatitis masquerading as a pancreatic neoplasm. Gastrointest Endosc 2008;68:796-8. [ Links ]
42. German V, Ekmektozoglou KA, Kyriakos N, et al. Pancreatitis of the gastroduodenal groove: A case report. Case Report Med 2010;2010:329587. doi:10.1155/2010/329587. [ Links ]
43. Ferreira A, Remalho M, Herédia V, et al. Groove pancreatitis: A case report and review of the literature. J Radiol Case Rep 2010;4:9-17. [ Links ]
44. Chute DJ, Stelow EB. Fine-Needle aspiration features of paraduodenal pancreatitis (groove pancreatitis): A report of three cases. Diagn Cytophathol 2011;40:1116-21. [ Links ]
45. Tyagi P, Thaper S, Bhatia V, et al. Often a missed type of pancreatitis: Groove pancreatitis. Indian J Gastroenterol 2012;31:215-6. [ Links ]
46. Goldaracena N, McCormack L. A typical feature of groove pancreatitis. HPB 2012;14:487-8. [ Links ]
47. Nankoe SR, Wilcox R, Roggin KK. Paraduodenal pancreatitis (groove pancreatitis) mimicking pancreatic adenocarcinoma. Clin Gastroenterol Hepatol 2012;10:A31-2. [ Links ]
48. Patriti A, Castellani D, Partenzi A, et al. Pancreatic adenocarcinoma in paraduodenal pancreatitis: A note of caution for conservative tretaments. Updates Surg 2012;64:307-9. [ Links ]
49. Lee TH, Park SH, Lee CK, et al. Ampulla of Vater metastasis from recurrent uterine cervix carcinoma presenting as groove pancreatitis. Gastrointest Endosc 2011;73:362-3. [ Links ]
50. Brosens LAA, Leguit RJ, Vleggaar FP, et al. EUS-guided FNA cytology diagnosis of paraduodenal pancreatitis (Groove pancreatitis) with numerous giant cells: Conservative management by cytological and radiological correlation. Cytopathology 2014. doi: 10.1111/cyt.12140 (Epub ahead of print). [ Links ]
51. Jiménez Fuertes M, Costa Navarro D. Distrofia quística de pared duodenal. De la incertidumbre diagnóstica a la confirmación anatomopatológica. Cir Esp 2014;92:498-9. [ Links ]
52. Dekeyzer S, Traen S, Smeets P. CT features of Groove pancreatitis subtypes. JBR-BTR 2013;96:365-8. [ Links ]
53. Yi PH, Veltre DR, Kuttab JS, et al. Acute Groove pancreatitis due to isoniazid. Neth J Med 2013;71:104. [ Links ]
54. Ciçek B, Ergüner I, Kara F, et al. Groove (paraduodenal) pancreatitis: Report of two cases. Turk J Gastroenterol 2013;24:173-7. [ Links ]
55. Irie H, Honda H, Kuroiwa T, et al. MRI of groove pancreatitis. J Comput Assist Tomogr 1998;22:651-5. [ Links ]
56. Castell-Monsalve FJ, Sousa-Martin JM, Carranza-Carranza A. Groove pancreatitis: MRI and pathologic findings. Abdom Imaging 2008; 33:342-8. [ Links ]
57. Levenick JM, Sutton JE, Smith KD, et al. Pancreaticoduodenectomy for the treatment of groove pancreatitis. Dig Dis Sci 2012;57:1954-8. [ Links ]
58. Kalb B, Martin DR, Sarmiento JM, et al. Paraduodenal pancreatitis: Clinical performance of MR imaging in distinguishing from carcinoma. Radiology 2013;269:475-81. [ Links ]
59. Zaheer A, Haider M, Kawamoto S, et al. Dual-phase CT finding of groove pancreatitis. Eur J Radiol 2014;83:1337-43. [ Links ]
60. Egorov VI, Vankovich AN, Petrov RV, et al. Pancreas-preserving approach to "paraduodenal pancreatitis" treatment: Why, when, and how? Experience of treatment of 62 patients with duodenal dystrophy. Biomed Res Int 2014;2014:185265. doi:10.1155/2014/185265. [ Links ]
61. Gabata T, Kadoya M, Terayama N, et al. Groove pancreatic carcinomas: Radiological and pathological findings. Eur Radiol 2003; 13:1679-84. [ Links ]
62. Shanbhogue AK, Fasih N, Surabhi VR, et al. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics 2009;29:1003-26. [ Links ]
63. Pezzilli R, Santini D, Calculli L, et al. Cystic dystrophy of the duodenal wall is not always associated with chronic pancreatitis. World J Gastroenterol 2011;17:4349-64. [ Links ]
64. Arora A, Dev A, Mukund A, et al. Paraduodenal pancreatitis. Clin Radiol 2014;69:299-306. [ Links ]
65. Perez-Johnston R, Sainani NI, Sahani DV. Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis). Radiol Clin N Am 2012;50:447-66. [ Links ]
66. Laugier R, Grandval P. Does paraduodenal pancreatitis systematically need surgery? Endoscopy 2014;46:588-90. [ Links ]
67. Blasbalg R, Baroni RH, Costa DN, et al. MRI features of groove pancreatitis. AJR Am J Roentgenol 2007;189:73-80. [ Links ]
68. Ishigami K, Tajima T, Nishie A, et al. Differential diagnosis of Groove pancreatic carcinomas vs groove pancreatitis: Usefulness of the portal venous phase. Eur J Radiol 2010;74:e95-100. [ Links ]
![]() Correspondence:
Correspondence:
José Manuel Ramia Ángel.
Hospital Universitario de Guadalajara.
C/ Donantes de Sangre, s/n.
19002 Guadalajara, Spain
e-mail:
jose_ramia@hotmail.com
Received: 01-04-2014
Accepted: 22-02-2015











 texto en
texto en