Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.5 Madrid may. 2015
LETTERS TO THE EDITOR
Induction of NKT cells after administration of a vaccine against rotavirus
Potente inducción de células NKT tras la administración de un preparado vacunal contra rotavirus
Key words: Vaccines against rotavirus. Rotarix™. CD3+/CD16+ CD56+ cells. NKT cells.
Palabras clave: Vacunas contra rotavirus. Rotarix™. Células CD3+/CD16+CD56+. Células NKT.
Dear Editor,
According to the World Health Organization and UNICEF, there are about two billion cases of diarrheal disease each year worldwide, this is the second cause of death in children under 5 years (after pneumonia), the problem seems to be extreme in children under one year of age (1), in which rotavirus infection is one of the most common causes of death.
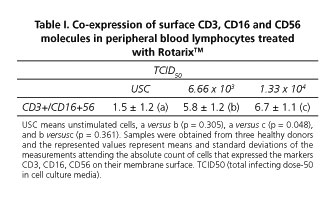
TM Rotarix (GSK Biologicals) vaccine consists of live attenuated human rotavirus, strain RIX4414, 106 TCID50 (total infecting dose-50 in cell culture media) and produced in VERO cells. In order to evaluate the effect of this vaccine preparation on the expression of molecules CD3+/CD16+CD56+ human cells, we proceeded to the isolation of mononuclear cells from peripheral blood of healthy donors using Ficoll-Paque™ PREMIUM, CA, USA gradient, density = 1.077. Once observed increased cell viability to 95% by count in hemocytometer with trypan blue dye; cells were added to flat botton- 96-well microtiter plaques (Sarstedt, Inc., Newton, NC 28658, USA), 3 x 105 cells/100 L per well. Cells were treated with Rotarix® (6.66 x 103 TCID50 g/mL or 1.33 x 104 TCID50 g/mL) and the control group did not receive the vaccine preparation. Subsequently, cells were incubated in a 37 oC, 5% CO2 in air, humidified environment for 6 hours (CO2 Incubator - SHEL LAB, USA). The number of CD3+/CD16+CD56+ was identified with a CD3/CD16 + CD56 antibody (CD3/CD16+CD56 FITC/PE -Beckman Coulter Inc., CA, USA-, in a xcl Epics flow cytometer. The student t test was used to compare means between treated cells and controls and a statistical significance of p < 0.05 was accepted.
The number of cells expressing CD3, CD16 and CD56 molecules on their membrane was 4.4 times higher in mononuclear cells treated with 1.33 x 104 TCID50 g/ml of the vaccine than those founded on untreated cells (p = 0.048) (Table I). These cells (NKT) have the ability to both: Constitutively as post-activation, promote a variety of immunoregulatory responses that lead to antiviral surveillance (2).
NKT cells have the advantage that when activated by the vaccine preparation can amplify both innate and adaptive response to promote critical events that occur within hours of exposure to viral antigens and promote resistance to virus (3).
It is known that NKT cells can be activated as dependent or independent of the antigen; this is an important way of amplification of the immune response, once the antigenic stimulus ceases; these cells also confer a functional plasticity to the immune response by its proinflammatory and immunoregulatory properties.
The contribution of the expansion of CD3+/CD16+CD56+ cells in peripheral blood on the effectiveness of the rotavirus vaccines need to be explore. The evaluation of the efficacy of the vaccine becomes more significant in high-mortality countries in which a reduced effectiveness of vaccine (4) could be associated to the earlier rotavirus exposure and a greater antigenic load of natural exposition to those observed in the rest of the countries. Two important tasks in these areas are the characterization of unusual genotypes that may be associated with decreased efficacy of the vaccine and the continuous monitoring of the prevalence of strains by each compromised region (5).
Alain R. Rodríguez-Orozco, Jesica Figueroa-Padilla,
Sergio Gutiérrez-Castellanos and Rocío del Carmen Montoya-Pérez
Facultad de Ciencias Médicas y Biológicas "Dr. Ignacio Chávez".
Universidad Michoacana de San Nicolás de Hidalgo. Morelia, México
References
1. Guía Práctica de la Organización Mundial de Gastroenterología. Diarrea aguda en adultos y niños: una perspectiva mundial. Febrero de 2012. Recuperado de http://www.worldgastroenterology.org/assets/export/userfiles/2012_Acute Diarrhea_SP.pdf. [ Links ]
2. van Dommelen SLH, Degli-Esposti, MA. NKT cells and viral immunity. Immunol Cell Biol 2004;82:332-41. [ Links ]
3. Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunology 2001;13:458-64. [ Links ]
4. Soares-Weiser K, Maclehose H, Bergman H, et al. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst Rev. 2012 Nov 14;11:CD008521. doi: 10.1002/14651858.CD008521.pub3. [ Links ]
5. Jain S, Vashistt J, Changotra H. Rotaviruses: Is their surveillance needed? Vaccine 2014;32:3367-78. [ Links ]











 texto en
texto en