My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.7 Madrid Jul. 2015
LETTERS TO THE EDITOR
Leiomyosarcoma of the inferior vena cava: Feasibility of surgical resection. A report of two cases
Key words: Inferior vena cava. Tumor resection. Metastasis. Adjuvant therapy.
Dear Editor,
Leiomyosarcoma of the inferior vena cava (IVC) is an extremely rare malignant tumor of the venous system and retroperitoneum (1). Retroperitoneal sarcomas may arise primarily from IVC or involve it secondarily (2). Due to its location, there is a lack of accurate and early symptoms, and these kinds of tumors are not diagnosed until the disease is at an advanced stage, with large tumor growth and involvement of surrounding structures. Clinical symptoms are unspecific, and for diagnosis, imaging tests such as color Doppler ultrasonography, contrast-enhanced computed tomography or magnetic resonance are required. Currently, to improve prognosis and provide long-term survival, radical en bloc/complete resection of the affected venous segment is the best treatment option (1,3,4). The goals of surgical procedure must include: Local tumor control, maintenance of cava flow, and the prevention of recurrence (4). The main classification used in the planning of a surgical approach divides the IVC into: An upper segment (above the hepatic veins), a middle segment (between hepatic and renal veins), and lower segment (below the renal veins). Furthermore, handling of the IVC after tumor resection is controversial, i.e. primary repair, ligation or reconstruction of the IVC have all been utilized with varying degrees of success (1). In this context, three important factors affect the need and the type of vascular replacement: a) The site of the lesion, and especially the involvement of renal veins; b) the extent of IVC resection (partial or circumferential); and finally c) the presence of a well-established collateral venous system (2).
We present two cases of leiomyosarcoma of the IVC, focusing on the type of surgical procedure and oncological outcome.
Case report 1
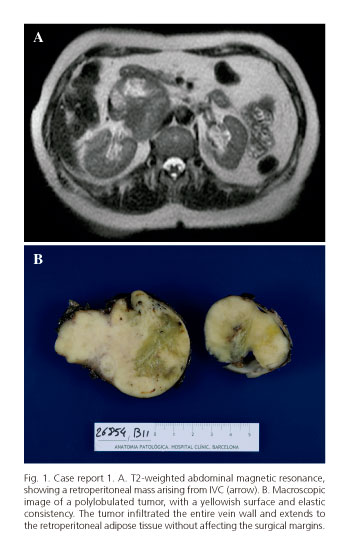
A 60-year-old female presenting with nonspecific abdominal pain was referred to our department for the treatment of a retroperitoneal tumor, detected through an abdominal ultrasonography. Although no abnormalities were apparent in the initial physical examination, a magnetic resonance and contrast-enhanced computed tomography imaging revealed a solid mass, 10x8 cm in size, below the hepatic hilum, consistent with a primary retroperitoneal tumor, most likely being a leiomyosarcoma of the IVC (Fig. 1A). Tumor markers were negative.
Upon a laparotomy investigation, a tumor originating from the wall of the IVC was apparent, extending from the infrahepatic portion to below the renal vein input. As the distal vena cava and left renal vein were thrombosed, the patient received intravenous heparin prior to exclusion of the venous segment. Significant retroperitoneal collateral venous circulation was evident. A tumor resection of the middle segment of the IVC, including the confluence and approximately two centimeters of the terminal left renal vein was performed. The tumor was then completely resected en bloc followed by an anastomosis between the infrahepatic vena cava and right renal vein. Blood loss amounted to 300 ml during the 150-minute operation time. Pathological diagnosis revealed a 7 cm high histological grade leiomyosarcoma, originating from the wall of IVC (Fig. 1B). Postoperative assessment of the surgical margins therefore confirmed that a complete R-0 resection had thus been achieved. No major incidents followed so the patient was discharged seven days postoperatively. No adjuvant treatment was administered at this moment. Nine months later, the patient showed pulmonary metastasis, receiving chemotherapy with doxorubicin. To date, 30 months after surgery, the pulmonary lesions are stable and show no signs of local recurrence.
Case report 2
A 65-year-old female patient presenting with hematuria and nonspecific abdominal pain underwent study. A contrast-enhanced computed tomography of the chest and abdomen revealed a large retroperitoneal heterogeneous mass, 7.0x6.5x9.4 cm in size (Fig. 2A). This endoluminal mass extended from the level of the IVC junction with the renal veins to the right atrium, and had extensive contact with both the posterior surface of the right hepatic lobe and the right kidney. An ultrasound-guided puncture revealed signs consistent with sarcoma. The echocardiogram showed a right atrial mass (3x4 cm) originating from IVC and extending toward the plane of the tricuspid valve, which was unaffected. Tumor markers were negative. The patient was operated through an abdominal and sternotomy approach with extracorporeal circulation and cannulation of left saphenous vein and pulmonary vein to optimize venous return. Furthermore, cannulation through a tributary of the portal system in the mesentery area was made to permit in situ liver perfusion with Ringer Lactate 4 oC. This procedure enables total hepatic vascular exclusion with clamping of the vena cava for 30 minutes in order to perform the retrohepatic vena cava resection. Following this, the vena cava was opened caudal to the tumor. Transesophageal ultrasound-guided confirmed removal of the entire intraluminal tumor reaching to the right atrium. Resection of upper and middle segments of the IVC was made, immediately cranial to the confluence of the left renal vein. As the tumor appeared to be infiltrating the right kidney, a right en bloc nephrectomy was performed. The IVC was replaced with a thoracic aorta graft. Total operating time was 360 minutes, with a blood loss totaling 925 ml. The pathological diagnosis was a 16x8 cm high histological grade leiomyosarcoma originating from the wall of IVC (Fig. 2B), and exhibiting intraluminal growth. There was no evidence of neoplasia at the edges of the surgical specimen. The kidney showed no tumor infiltration. Postoperatively, the patient developed a subhepatic hematoma that was managed conservatively. The patient was discharged fourteen days from surgery without oral anticoagulation. It was further decided that the patient would not receive chemotherapy. At follow-up after 6 months of surgery, the patient had pulmonary and hepatic metastasis, receiving ifosfamide. Two years after surgery, metastasis lesions are stable and the patient is alive.
Discussion
The current treatment of choice for leiomyosarcoma of the IVC continues to be radical surgery with negative margins (3,5). In fact, it constitutes the only hope of prolonged survival, taking into consideration that neither radiotherapy nor chemotherapy are, as yet, effective (1,4,5).The value of preoperative treatments in resectable tumors or adjuvant chemotherapy, is not established (5). In general, post-operative radiation therapy to the whole tumor bed at doses recommended for sarcomas is not feasible at currently acceptable levels of toxicity (5). Therefore, the literature recommends aggressive surgical management through the use of modern vascular surgical and oncological techniques (4,5). However, recurrence occurs in approximately 50% of the patients who undergo curative resection. Literature has reported actuarial survival rates at 5 years of 49.4%, with cancer free actuarial survival rates at 5 and 10 years of 31.4% and 7.4%, respectively (1,3).
Surgical procedures for IVC leiomyosarcoma extirpation are frequently complex due to the retro hepatic location and close proximity to major branches of the IVC (1). Regarding the technique, surgeons have several options depending on the location of the tumor in the IVC and its extension, which includes mainly the ligation of the vein, cavoplasty, and graft replacement (1,2). Our cases have required ligation in the former case, and aorta allograft in the latter. For the first patient, ligation was considered intraoperative because of the discovery of an important collateral circulation that provided a sufficient venous drainage for the left renal and iliac veins due to the chronic thrombosis of the distal IVC. After surgery, no vascular problem was observed. For the second patient, the decision was made preoperatively due to the extension of the tumor. Concerning allografts, there are some considerations in the literature; for example, a PTFE prosthesis or banked venous/artery allograft could be used. With this approach, the most common complications are infection and graft occlusion, occurring in 7 to 28% (2), although not so in the included cases. Contraindications to surgical resection include the presence of widespread metastases, involvement of major vascular structures such as celiac and superior mesenteric arteries, the portal vein and the superior mesenteric vein (1).
To conclude, we believe that although this pathology requires aggressive surgical techniques, it is important to grant the patient the possibility of an oncologic surgical resection (R0), which is associated with improved survival. The role of adjuvant therapy is still undecided.
Luis Flores1, Joana Ferrer1, Mario Pages2, Josep Ramírez3,
Josep Fuster1 and Juan Carlos García-Valdecasas1
1Hepato-Bilio-Pancreatic Surgery and Transplantation Unit. Institut
de Malalties Digestives i Metabòliques (ICMDiM). Departments of
2Radiology and 3Pathology. Hospital Clínic. Barcelona, Spain
References
1. Cho SW, Marsh JW, Geller DA, et al. Surgical management of leiomyosarcoma of the inferior vena cava. J Gastrointest Surg 2008;12:2141-8. DOI: 10.1007/s11605-008-0700-y. [ Links ]
2. Fiore M, Colombo C, Locati P, et al. Surgical technique, morbidity, and outcome of primary retroperitoneal sarcoma involving inferior vena cava. Ann Surg Oncol 2012;19:511-8. DOI: 10.1245/s10434-011-1954-2. [ Links ]
3. Kieffer E, Alaoui M, Piette JC, et al. Leiomyosarcoma of the inferior vena cava: Experience in 22 cases. Ann Surg 2006;244:289-95. DOI: 10.1097/01.sla.0000229964.71743.db. [ Links ]
4. Alexander A, Rehders A, Raffel A, et al. Leiomyosarcoma of the inferior vena cava: Radical surgery and vascular reconstruction. World Journal of Surgical Oncology 2009;7:56. DOI: 10.1186/1477-7819-7-56. [ Links ]
5. Blay JY, Blomqvist C, Bonvalot S, et al. ESMO / European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Surg Oncol 2012;7:92-9. [ Links ]