Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.8 Madrid ago. 2015
Management of fundic varices. Endoscopic aspects
Javier Martínez-González, Sergio López-Durán, Enrique Vázquez-Sequeiros and Agustín Albillos-Martínez
Department of Gastroenterology and Hepatology. Hospital Universitario Ramón y Cajal, IRYCIS. Madrid, Spain
Background
Gastric varices (GV) are less prevalent than esophageal ones. It has been estimated that GV are present in 8-15% of patients with cirrhosis and portal hypertension. However, the prevalence of GV, in those patients with non-cirrhotic portal hypertension, has been estimated to be around 20%. GV should also be investigated in patients who present with splenic vein thrombosis (segmentary portal hypertension) (1-3). Although gastrointestinal bleeding from GV is less frequent than from esophageal varices, bleeding from GV is usually more severe (increased rate of morbidity, mortality, rebleeding and transfusion requirements) (1,4,5).
Gastric varices classification
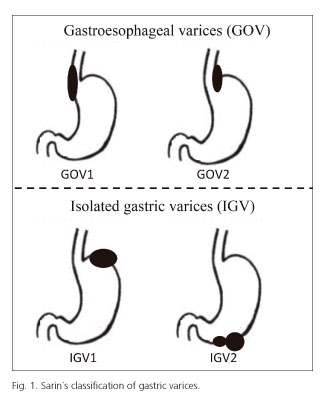
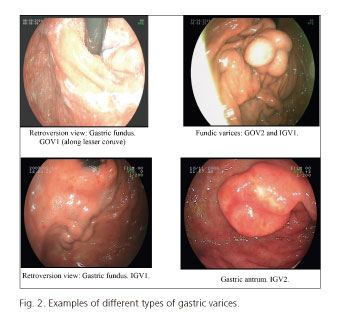
Sarin's classification divides GV according to their location in the stomach, as gastroesophageal varices (GOV) and isolated gastric varices (IGV) (Figs. 1 and 2). This classification is the most frequently used in clinical practice, as it correlates each type of varices with a specific risk of bleeding, modality of treatment, and vascular anatomy (4,5).
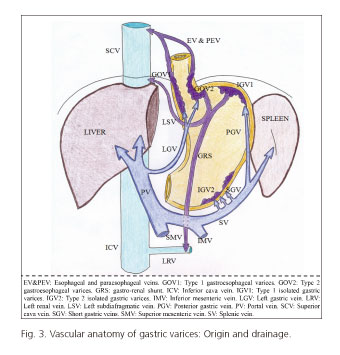
GOV are divided into 2 subtypes. GOV1 are gastric varices arising from the lesser curvature of the stomach and extending above the gastroesophageal junction as esophageal varices. GOV2 varices are located in the gastric fundus and extend towards the esophagus as esophageal varices. Both of them, GOV1 and GOV2, arise from the left gastric vein. GOV1 drain into the azygos vein and superior vena cava through the esophageal and paraesophageal veins. GOV2 follow the same route as GOV1 varices, but they also drain to the inferior vena cava vein through the subdiafragmatic left vein. GOV1 are the most common type of GV (70% of the total), but have a lower risk of bleeding (responsible for only 20% of GV bleedings). On the other hand, GOV2 represent only the 21% of all GV. IGV are even less common, and can be located in fundus (IGV1) (7%) or in the body or antrum of the stomach (IGV2) (2%), with no extension into the esophagus (4). IGV arise from the short gastric veins and the posterior gastric vein (both of them, collaterals from splenic vein), and may drain through the left subdiafragmatic vein, laterally to the inferior vena cava (gastro-cava shunt) or drain down to the left renal vein (gastro-renal shunt) (5). Figure 3 shows the vascular anatomy of gastric varices.
GOV2 and IGV1, but not IGV2, are both located in the gastric fundus, and therefore are named as fundic varices. Eighty-five per cent of patients who present with fundic varices will have a gastro-renal shunt (from the fundus to the left renal vein), which may be responsible for many physiological and therapeutic aspects of fundic varices (6-8).
Vascular anatomy and management of GOV1 and esophageal varices, as well as treatment response is similar, and therefore will not be discussed in this review. Although only 30% of all GV are located in the gastric fundus, they are responsible for nearly 70% of all GV bleeding (4). This review will focus on fundic varices management.
Treatment of bleeding from fundic varices
Primary prophylaxis
Risk factors associated with bleeding from fundic varices were analyzed by Kim et al. (9). Authors observed that the degree of liver function (as measured by the Child-Pugh and/or MELD score), the size of the varix and the presence of red spots on its surface were all associated with the risk of bleeding (9). The international consensus on portal hypertension management held in 2010 (Baveno V) recommends the usage of non-selective beta blockers for primary prophylaxis in patients with GV (10). However, in 2011 Sarin et al. (11) conducted a randomized trial comparing three different strategies of GV therapy (cyanoacrylate injection vs. non-selective beta blockers vs. no treatment). Significant differences were observed favouring the use of cyanocrylate (CA) injection, in terms of prevention of bleeding and survival, when compared with no treatment. However, when compared with propranolol, CA only showed significant benefits in preventing the first bleeding. This study has been criticized due to important limitations, as highlighted by Tripathi (12): a) Alcohol was the only known etiology (nearly 50% of patients), with the remaining patients being classified as idiopathic (26%) or unknown (26%); b) most of the patients included in the study had GOV2 varices with a very small number of IGV1 cases; and c) results of the hepatic venous pressure gradient were heterogeneous, with a wide range of measures and some cases having less than 10 mmHg of gradient. The last international consensus on portal hypertension management held in 2015 (Baveno VI) (13), advocates that further studies are needed to evaluate the risk/benefit ratio of using CA in this setting before a recommendation can be made.
Due to these study limitations and the little scientific evidence for primary prophylaxis of GV, the Spanish consensus document, sponsored by the Spanish Association for the Study of the Liver (AEEH), recommend the use of beta-blockers (5;D), and to avoid CA injection for primary prophylaxis (3). However the previously cited study by Sharin et al. (11) may be taken into account in the next AEEH recommendations.
Treatment of acute bleeding from fundic varices
There is no strong evidence regarding which therapy should be performed in patients with an episode of acute bleeding from fundic varices. The initial approach for these patients is, as in esophageal varices bleeding (1,6,10,14), to initiate therapies directed to obtain patient hemodynamic stability, administer vasoactive drugs (somatostatin, terlipressin) and antibiotics, and transfuse blood as required (following a restrictive policy).
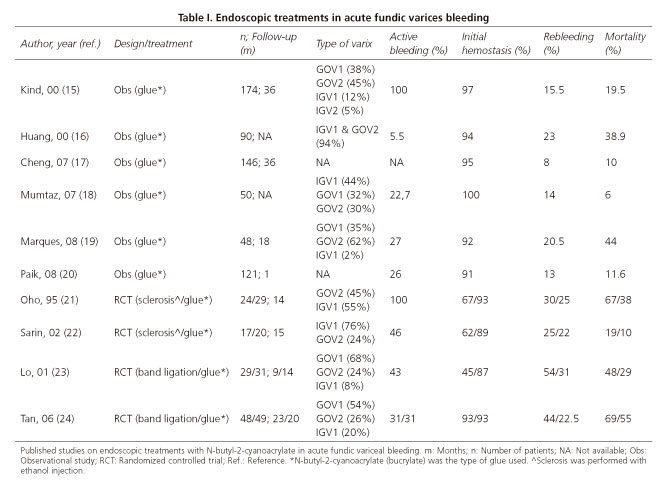
If endoscopic therapy is advised, we should know that there are three alternatives for such a treatment: CA injection, endoscopic band ligation and ethanol injection. The scientific evidence supporting the use of endoscopic therapy to treat these patients is limited, as most studies published include not only fundic varices and this may certainly limit the validity of conclusions obtained. Careful review of studies published on the literature, shows that the best endoscopic option for treating fundic varices that are actively bleeding is CA injection (Table I), with a reported technical success of approximately 90% (5,15-24). Endoscopic band ligation may also be consider as an alternative, but should only be applied in selected cases of actively bleeding GOV2 of small size, and when the endoscopist has low experience with CA injection. In some studies endoscopic band ligation has been shown to achieve an elevated rate of hemostasis, similar to CA injection, but the elevated rate of rebleeding (close to 50% in some publications) does not allow us to recommend it as a first line therapy (23,24). Ethanol injection of bleeding gastric varices has also been reported, but existing evidence does not support its use, as it shows a lower efficacy and a higher complication rate than other endoscopic alternatives (21,22).
In those cases of active, massive, uncontrollable bleeding, balloon tamponade with the Sengstaken or Linton balloon appears to be the best therapeutic option. The efficacy and safety of both types of balloon in this setting has only been compared in a single study. It does appear that the Linton balloon, that is provided with a larger gastric balloon (600 ml of volume) than the Sengstaken balloon (400 ml), is probably more effective to obtain adequate mechanic compression of the fundic varices (25). Balloons have been shown to be very effective to achieve initial haemostasis (80%), but tend to rebleed after deflation (> 50%), and therefore should only be considered as a temporal bridge (24-48 h) to definitive therapy. After deflating the balloon, one of the following therapeutic options should be performed to prevent bleeding: CA injection, transjugular intrahepatic portosystemic shunt (TIPS), balloon-occluded retrograde transvenous obliteration (BRTO), or surgery for selected cases (1,2,6). Surgical aspects are beyond the focus of this revision (endoscopic therapy of gastric varices), and will not be discussed in this manuscript.
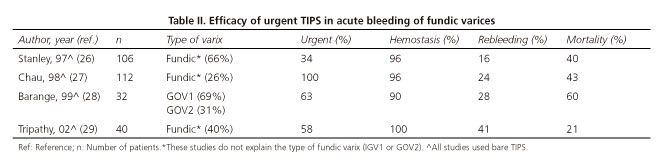
Placement of a TIPS has been shown to be very effective at controlling the episode of active bleeding, achieving an hemostasic rate as high as 90-100%, with a moderate risk of rebleeding (16-41%) (26-29) (Table II). Generally speaking, the TIPS should be considered if the bleeding is not controlled after CA injection, or at the time of balloon deflation (27,30). Treatment of collateral veins visualized by portography during the TIPS placement is not routinely performed; however, it has been recommended in the following situations: a) Venous collaterals seen on portography after TIPS placement; b) rebleeding from fundic varices after successful TIPS insertion; and c) venous gradient > 12 mmHg after TIPS placement (3,31,32). Contraindications for TIPS should be carefully studied in a case by case scenario, considering the patient clinical status (degree of liver dysfunction and portal hypertension: Bilirubin, albumin, platelets, sodium, past history of encephalopathy) and technical aspects like vessel permeability (29).
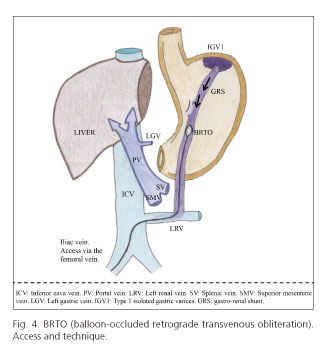
BRTO is a novel technique developed in Japan and performed in a few centers in the USA. It does appear to have a high technical success and efficacy (75-100%), with a low rebleeding rate (0-15%) (33). From a technical point of view, it should be acknowledged that a wide gastro-renal shunt is required to be able to reach the fundic varices from left renal vein, inflate the balloon inside the shunt, and finally inject CA to occlude the gastric varices (Fig. 4). BRTO has been mostly employed as a primary prophylaxis method; however, in one single study, BRTO was shown to achieve a high haemostatic rate after controlling the initial bleeding episode with a balloon tamponade (33).
In summary, it does appear that the best therapeutic options for actively bleeding gastric fundic varices are CA injection and TIPS (9,31,32). The international consensus on portal hypertension held in 2015 (Baveno VI) recommended CA injection as the first therapeutic option (1;A) (10), while TIPS should be considered as the second option, and indicate it only when CA injection fails or after balloon deflation (2;B) (30,31).
Secondary prophylaxis
It has been estimated that the rebleeding rate of fundic varices is close to 15% (1-3). Some studies have demonstrated that CA injection is the most effective endoscopic technique for secondary prophylaxis, superior to endoscopic band ligation and/or ethanol injection (3,6). The usefulness of non-selective beta blockers in this setting remains controversial. In a clinical trial comparing CA injection and non-selective beta blockers for secondary prophylaxis of fundic varices, CA injection proved to be more effective in terms of rebleeding and overall survival (34). In another study, authors demonstrated that the addition of non-selective beta blockers to CA injection for secondary prophylaxis does not appear to reduce rebleeding rate or improve survival (35). However, other reports have shown a better survival in those patients treated with the combination of CA injection and a non-selective beta blocker (36).
On the other hand, there is strong evidence supporting the use of TIPS for secondary prophylaxis (as well as CA injection) (1,6). However, studies evaluating the efficacy of TIPS for this indication did not include patients with fundic varices. The TIPS has shown a high efficacy and a similar rebleeding rate than CA injection, with no significant differences in terms of survival (37,38). As TIPS may associate a greater morbidity than CA injection (37), and as shown in one study CA injection is probably more cost-effective (38), there is an important question that remains unanswered: Should we indicate a TIPS to all patients that have a history of bleeding from fundic varices, or on contrary we should only offer this alternative to those patients in whom CA injection has already failed (3).
The international consensus on portal hypertension of the year 2015 (Baveno VI) (13) recommends that to prevent rebleeding from gastric varices, consideration should be given to additional glue injection (after 2 to 4 weeks), beta-blocker therapy or both combined, or TIPS. Other options of therapy may include BRTO (in case of TIPS contraindication or technical difficulties) (40,41), or derivative surgery, but these therapeutic alternatives should be carefully considered in a case by case approach, depending on patient characteristics (degree of liver dysfunction) and surgeon skills (1,3).
Technical aspects of cyanoacrylate injection
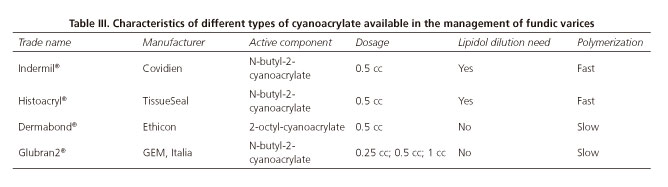
Among other potential indications for glue injection in the field of Gastroenterology, the most useful application of this type of therapy is the treatment of fundic gastric varices (42). There are different types of glues (Table III), but all of them have in common that CA is part of their chemical composition. CA posses the characteristic of becoming solid very quick when it gets in contact with a weak base substance, like water or blood. Different trademarks of CA are manufactured by different companies. The differences among them are mainly based on chemical variations in the length of alkyl group, which may alter the physicochemical properties of the agent. For this reason, some of these glues (Indermil® and Histoacryl®) have to be diluted with lipiodol in a ratio of 1:1 or 1:1.6, to reduce the polymerization speed. However other types of CA (Dermabon® and Glubran 2®) have longer polymerization times and therefore will not require dilution with lipiodol, being in our opinion easier to use (42,43). In cases in which CA is diluted with lipiodol, it should be recommended to monitor the injection by radiologic control.
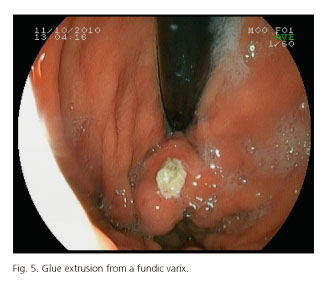
The reported rate of obliteration of fundic varices after CA injection is high (90%), and the rebleeding rate oscillates between 0-15% (15,16). CA injection is a relatively safe technique; however, some reports have described a mortality of 0.5% (44). Additionally, it should be mentioned that a number of adverse effects have been described with CA injection: Chest pain, fever, rebleeding due to CA extrusion (Fig. 5), splenic infarction, portal and splenic veins thrombosis, sepsis, fistulae, etc. One of the most feared adverse effects associated with this type of treatment is the embolization of the CA injected. In a study in which a CT-scan was systematically performed after CA injection, results disclosed that 47% of patients had pulmonary embolisms of CA, but only 1% was symptomatic. If the patient has arteriovenous pulmonary shunts or a patent foramen oval, systemic embolization to other distant regions may occur (42-46). A number of factors have been associated with an increased risk of embolization: a) Injection in IGV1; b) rapid injection; and c) overdilution with lipiodol (43).
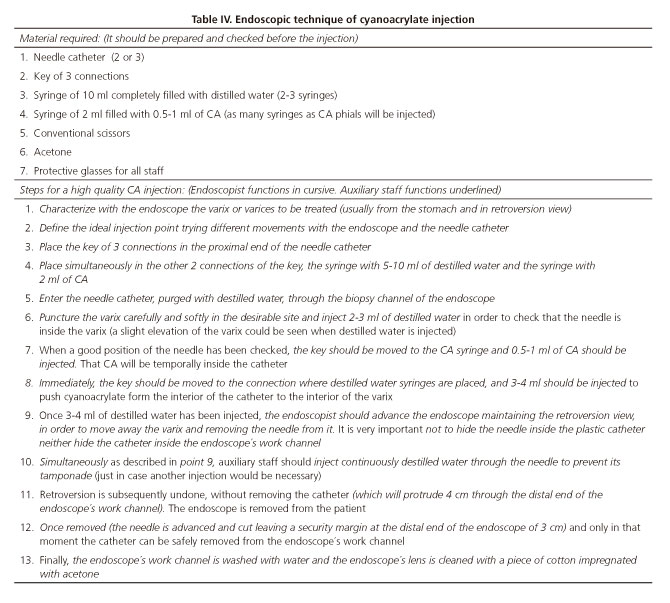
We believe that to do the best work at CA injection, one should have an adequate understanding of the indications, be familiar with the technique and with the type of CA used, and have a good coordination with the auxiliary personnel. On table IV, a detailed description of every step required for CA injection is shown. As we do in our general practice, we recommend the use of a CA type that does not require dilution with lipiodol.
After retrieval of the needle from the varix, a back-bleeding may be observed. Before doing another injection to achieve hemostasia, we recommend to wait and observe for a while, as CA haemostatic effect is not instantaneous. The majority of studies published in the literature have employed dosages of 0.5-1 ml of CA, without observing clinical differences between dosages. Although there is no supporting evidence for such a practice, most authors will recommend, for safety reasons (systemic embolization risk), not to use more than 1-2 ml of CA per session (47,48). There are still some questions that need to be answered, and hopefully we will have definitive answers for them in coming years: a) Which type of CA is the most effective?; b) what should be the ideal amount/volume of CA that should be injected per varix and session?; c) in case of using a type of CA with fast polymerization, which dilution rate would be the best one?; d) should intravascular injection of CA be checked by radioscopy or with an echoendoscope?; and e) what is the best way to confirm that the varix has been obliterated (gentle pressure with a plastic catheter vs. endoscopy-ultrasound with Doppler), and how much time should we wait before doing it?
Finally, it should be mentioned that there are other sclerosing agents, like thrombin or fibrinogen that may potentially be as efficacious as CA. Unfortunately, little information is available in the literature, preventing from widespread use of them in clinical practice.
Endoscopic ultrasound (EUS) in fundic varices
The diagnosis of gastric fundic varices by means of standard upper gastrointestinal endoscopes may be sometimes really difficult, mainly in those cases of small size or by misdiagnosing them as thickened gastric folds. In those uncertain cases, EUS with the help of the Doppler function might be helpful to further clarify the diagnosis, proving a definitive diagnosis if intravariceal flow or venous flow is demonstrated (43).
EUS has been proposed as having some theoretical advantages for CA injection. EUS allows one for real-time confirmation of appropriate delivery of CA into the varix lumen, permits to monitor the entrance of the CA in the varix, and it may be performed even in those cases in which there is no option to obtain a direct endoscopic visualization of the varix for injection. Moreover, it has been postulated that the identification and selective injection of CA in the main perforator feeding vein may reduce the amount of glue needed to achieve varix obliteration, reducing the potential risk of embolization. However, this theoretical advantage has not been adequately demonstrated and, from a practical point of view, it may be technically challenging (44,49).
Other authors have proposed that EUS may also allow one to inject CA in fundic varices through a transesophageal approach (in those cases with no esophageal varices). This may avoid retroflex therapy of fundic varices that in certain cases may be difficult or not technically possible. This technique would also avoid back-bleeding when removing the needle from the varix as it has been postulated that the diaphragm and the esophagus wall would act as a compressive barrier for the varix (50). However, this theoretical advantage of EUS is yet to be demonstrated.
Recent publications have shown that the combination of EUS-guided CA injection in conjunction with intravarix coil deployment may be technically feasible. Initial coil placement may theoretically produce a partial thrombosis of the varix, with the intention of requiring a smaller amount of CA to achieve a complete obliteration of the gastric varix afterwards. This novel combined approach has been shown, in preliminary reports, to have a good efficacy and a reasonable complication rate (48-50). However, more scientific evidence is still required before one may confidently recommend its use in clinical practice.
Despite the aforementioned theoretical advantages of EUS guided treatment of gastric varices (injection of CA in the perforator feeding veins, use of coils combined with CA, possibility of using a transesophageal approach for CA injection), and based on our clinical experience and existing evidence, we believe that the only demonstrated utility of EUS in the treatment of fundic varices is to confirm its diagnosis in uncertain cases after endoscopy, and to monitor (with the Doppler) if the varix has been obliterated after CA injection or a second session is required. It is our believe that other indications proposed for EUS in this setting should be considered investigational at present time.
References
1. Tripathi D, Ferguson J, Therapondos G, et al. Review article: Recent advances in the management of bleeding gastric varices. Aliment Pharmacol Ther 2006;24:1-17. [ Links ]
2. Hashizume M, Akahoshi T, Tomikawa M. Management of gastric varices. J Gastroenterol Hepatol 2011;26:102-8. [ Links ]
3. Bosch J, Abraldes J, Albillos A, et al. Hipertensión portal: Recomendaciones para su evaluación y tratamiento. Documento de consenso auspiciado por la AEEH y el CIBERehd. Gastroenterol Hepatol 2012;35:421-50. [ Links ]
4. Sarin S, Lahoti D, Saxena S, et al. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology 1992;16:1343-9. [ Links ]
5. Kiyosue H, Ibukuro K, Maruno M, et al. Multidetector CT anatomy of drainage routes of gastric varices: A pictorial review. Radiographics 2013;33:87-100. [ Links ]
6. Turon F, Casu S, Hernández-Gea V, et al. Variceal and other portal hypertension related bleeding. Best Pract Res Clin Gastroenterol 2013;27:649-64. [ Links ]
7. Watanabe K, Kimura K, Matsutani S, et al. Portal hemodynamics in patients with gastric varices: A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology 1988;95:434-0. [ Links ]
8. Matsumoto A, Kitamoto M, Imamura M, et al. Three-dimensional portography using multislice helical CT is clinically useful for management of gastric fundic varices. Am J Roentgenol 2001;176:899-05. [ Links ]
9. Kim T, Shijo H, Kokawa H, et al. Risk factors for hemorrhage from gastric fundal varices. Hepatology 1997;25:307-12. [ Links ]
10. Franchis R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-8. [ Links ]
11. Mishra S, Sharma B, Kumar A, et al. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: A randomized controlled trial. J Hepatol 2011;54:1161-7. [ Links ]
12. Tripathi D. Primary prophylaxis against gastric variceal bleeding: Is there a sticky solution at last? Hepatology 2011;54:1994-6. [ Links ]
13. Franchis R. Expanding consensus in portal hypertension: Report of Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015. Accepted. In press. [ Links ]
14. Garcia-Tsao G, Sanyal A, Grace N, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922-38. [ Links ]
15. Kind R, Guglielmi A, Rodella L, et al. Bucrylate treatment of bleeding gastric varices: 12 years' experience. Endoscopy 2000;32:512-9. [ Links ]
16. Huang Y, Yeh H, Chen G, et al. Endoscopic treatment of bleeding gastric varices by N-butyl-2-cyanoacrylate (Histoacryl) injection: Long-term efficacy and safety. Gastrointest Endosc 2000;52:160-7. [ Links ]
17. Cheng LF, Wang ZQ, Li CZ, et al. Treatment of gastric varices by endoscopic sclerotherapy using butyl cyanoacrylate: 10 years' experience of 635 cases. Chin Med J 2007;120:2081-5. [ Links ]
18. Mumtaz K, Majid S, Shah H, et al. Prevalence of gastric varices and results of sclerotherapy with N-butyl 2 cyanoacrylate for controlling acute gastric variceal bleeding. World J Gastroenterol 2007;13:1247-51. [ Links ]
19. Marques P, Maluf-Filho F, Kumar A, et al. Long term outcomes of acute gastric variceal bleeding in 48 patients following treatment with cyanoacrylate. Dig Dis Sci 2008;53:544-50. [ Links ]
20. Paik C, Kim S, Lee I, et al. The therapeutic effect of cyanoacrylate on gastric variceal bleeding and factors related to clinical outcome. J Clin Gastroenterol 2008;42:916-22. [ Links ]
21. Oho K, Iwao T, Sumino M, et al. Ethanolamine oleate versus butyl cyanoacrylate for bleeding gastric varices: A nonrandomized study. Endoscopy 1995;27:349-54. [ Links ]
22. Sarin S, Jain A, Jain M, et al. A RCT of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. Am J Gastroenterol 2002;97:1010-5. [ Links ]
23. Lo G, Lai K, Cheng J, et al. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology 2001;33:1060-4. [ Links ]
24. Tan P, Hou M, Lin H, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2- cyanoacrylate injection versus band ligation. Hepatology 2006;43:690-7. [ Links ]
25. Terés J, Cecilia A, Bordas J, et al. Esophageal tamponade for bleeding varices. Controlled trial between the Sengstaken-Blakemore tube and the Linton-Nachlas tube. Gastroenterology 1978;75:566-9. [ Links ]
26. Stanley AJ, Jalan R, Ireland HM, et al. A comparison between gastric and oesophageal variceal haemorrhage treated with TIPS. Aliment Pharmacol Ther 1997;11:171-6. [ Links ]
27. Chau T, Patch D, Chan Y, et al. "Salvage" transjugular intrahepatic portosystemic shunts: Gastric fundal compared with esophageal variceal bleeding. Gastroenterology 1998;114:981-7. [ Links ]
28. Barange K, Péron J, Imani K, et al. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology 1999;30:1139-43. [ Links ]
29. Tripathi D, Therapondos G, Jackson E, et al. The role of the transjugular intrahepatic portosystemic shunt (TIPS) in the management of bleeding gastric varices: Clinical and haemodynamic correlations. Gut 2002;51:270-4. [ Links ]
30. Azoulay D, Castaing D, Majno P, et al. Salvage transjugular intrahepatic portosystemic shunt for uncontrolled variceal bleeding in patients with decompensated cirrhosis. J Hepatol 2001;35:590-7. [ Links ]
31. Xiao T, Chen L, Chen W, et al. Comparison of TIPS alone Versus TIPS combined with embolotherapy in advanced cirrhosis: A retrospective study. J Hepatol 2001;35:590-7. [ Links ]
32. Gaba RC, Bui JT, Cotler SJ, et al. Rebleeding rates following TIPS for variceal hemorrhage in the Viatorr era: TIPS alone versus TIPS with variceal embolization. Hepatol Int 2010;4:749-56. [ Links ]
33. Choi Y, Yoon C, Park J, et al. BRTO for gastric variceal bleeding: Its feasibility compared with TIPS. Korean J Radiol 2003;4:109-16. [ Links ]
34. Mishra S, Sharmar B, Kumar A, et al. Endoscopic cyanoacrylate injection versus beta-blocker for secondary prophylaxis of gastric variceal bleed: A randomised controlled trial. Gut 2010;59:729-35. [ Links ]
35. Hung H, Chang C, Hou M, et al. Efficacy of non-selective beta-blockers as adjunct to endoscopic prophylactic treatment for gastric variceal bleeding: A randomized controlled trial. J Hepatol 2012;56:1025-32. [ Links ]
36. Choi M, Kin Y, Kim S, et al. The secondary prophylactic efficacy of beta-blocker after endoscopic gastric variceal obturation for the first acute episode of gastric variceal bleeding. Clin Mol Hepatol 2013;19:280-7. [ Links ]
37. Procaccini N, Al-Osaimi A, Northup P, et al. Endoscopic cyanoacrylate versus TIPS for gastric variceal bleeding: A single-center U.S. analysis. Gastrointest Endosc 2009;70:881-7. [ Links ]
38. Lo G, Liang H, Chen W, et al. A prospective, randomized controlled trial of TIPS versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy 2007;39:679-85. [ Links ]
39. Mahadeva S, Bellamy M, Kessel D, et al. Cost-effectiveness of N-butyl-2-cyanoacrylate (histoacryl) glue injections versus TIPS in the management of acute gastric variceal bleeding. Am J Gastroenterol 2003;98:2688-93. [ Links ]
40. Kanagawa H, Mima S, Kouyama H, et al. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol 1996;11:51-8. [ Links ]
41. Caldwell S. Gastric varices: Is there a role for endoscopic cyanoacrylates, or are we entering the BRTO era? Am J Gastroenterol 2012;107:1784-90. [ Links ]
42. Cameron R, Binmoeller K. Cyanoacrylate applications in the GI tract. Gastrointest Endosc 2013;77:846-57. [ Links ]
43. Bhat Y, Banerjee S, Barth B, et al. Tissue adhesives: Cyanoacrylate glue and fibrin sealant. Gastrointest Endosc 2013;78:209-15. [ Links ]
44. Cheng L, Wang Z, Li C, et al. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clin Gastroenterol Hepatol 2010;8:760-6. [ Links ]
45. Levy M, Wong L. EUS-guided angiotherapy for gastric varices: Coil, glue, and sticky issues. Gastrointest Endosc 2013;78:722-4. [ Links ]
46. Hou M, Lin H, Lee H, et al. A randomized trial of endoscopic cyanoacrylate injection for acute gastric variceal bleeding: 0.5 mL versus 1.0mL. Gastrointest Endosc 2009;70:668-75. [ Links ]
47. Seewald S, Ang T, Imazu H, et al. A standardized injection technique and regimen ensures success and safety of cyanoacrylate injection for the treatment of gastric fundal varices. Gastrointest Endosc 2008;68:447-54. [ Links ]
48. Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, et al. Endoscopic ultrasound (EUS)-guided therapy of gastric varices: Results from a prospective multicenter study. Gastrointest Endosc 2013;78:711-21. [ Links ]
49. Binmoeller K, Weilert F, Shah J, et al. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection. Gastrointest Endosc 2011;74:1019-25. [ Links ]
50. Romero-Castro R, Pellicer-Bautista F, Giovannini M, et al. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy 2010;42:E35-6. [ Links ]
![]() Correspondence:
Correspondence:
Javier Martínez-González.
Department of Gastoenterology and Hepatology.
Hospital Universitario Ramón y Cajal, IRYCIS.
Ctra. de Colmenar, km. 9.100.
28034 Madrid, Spain
e-mail: martinez.gonzalez.javier@gmail.com
Received: 28-08-2014
Accepted: 06-02-2015