Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.10 Madrid oct. 2015
Screening of enzymatic synthesis and expression of Lewis determinants in human colorectal carcinoma
Almudena Fernández-Briera1, Elisa Cuevas2 and Emilio Gil-Martín1
1 Department of Biochemistry, Genetics and Immunology. Faculty of Biology. University of Vigo. Vigo, Spain.
2 Pathology Service. Complejo Hospitalario Universitario. Ourense, Spain
This study was funded by the "Contrato-Programa de Consolidación y Estructuración de Unidades de Investigación Competitivas", ref. no. CN 2011/024, Xunta de Galicia, Spain.
ABSTRACT
Background: Although colorectal carcinogenesis has been intensively studied, the published investigations do not provide a consistent description of how different carbohydrate determinants of colorectal epithelium are modified in colorectal cancer (CRC).
Objective: This study is an attempt to characterize the terminal fucosylation steps responsible for the synthesis of mono-(Lea/Lex) and difucosylated (Leb/Ley) Lewis antigens in healthy and tumour CRC tissue.
Methods: An immunohistochemical study of Lewis antigens' expression was undertaken, along with screening of the fucosyltransferase (FT) activities involved in their synthesis, on healthy and tumour samples from 18 patients undergoing CRC.
Results: Analysis of α(1,2/3/4)FT activities involved in the sequential fucosylation of cores 1 and 2 showed significant increases in tumour tissue. Expressed as μU/mg and control vs. tumour activity (p from Wilcoxon's test), the FT activities for Lea/Leb synthesis were: lacto-N-biose α(1,2)/α(1,4)FT, 65.4 ± 19.0 vs. 186 ± 35.1 (p < 0.005); lacto-N-fucopentaose 1 α(1,4)FT, 64.9 ± 11.9 vs. 125.4 ± 20.7 (p < 0.005); Lea α(1,2)FT, 56.2 ± 7.2 vs. 130.5 ± 15.6 (p < 0.001). Similarly, for Lex/Ley synthesis were: N-acetyllactosamine α(1,2)-/α(1,3)FT, 53.4 ± 12.2 vs. 108.1 ± 18.9 (p < 0.001); 2'-Fucosyl-N-acetyllactosamine α(1,3)FT, 61.3 ± 10.7 vs. 126.4 ± 22.9 (p < 0.001); 2'-Fucosyllactose α(1,3)FT, 38.9 ± 10.9 vs. 143.6 ± 28.9 (p < 0.001); 2'-Methyllactose α(1,3)FT, 30.9 ± 4.8 vs. 66.1 ± 8.1 (p < 0.005); and Lex α(1,2)FT, 54.3 ± 11.9 vs. 88.2 ± 14.4 (p < 0.001).
Immunohistochemical Ley expression was increased (p < 0.01 according to Wilcoxon's test) in tumour tissue, with 84.6% of specimens being positive: 7.7% weak, 15.4% moderate and 61.5% high intensity.
Conclusions: Results suggest the activation of the biosynthesis pathways of mono-and difucosylated Lewis histo-blood antigens in tumour tissue from CRC patients, leading to the overexpression of Ley, probably at the expense of Lex.
Key words: Fucosyltransferases. Colorectal cancer. Lea. Leb. Lex. Ley.
Introduction
Conventional staging methods of colorectal cancer (CRC) are not efficient enough and fail to correct prediction of tumour progression. Therefore, it is necessary to further research on molecular and structural biomarkers of cancer cells, which may provide a better indication of malignancy evolution than staging alone, and could improve the clinical practice of CRC selecting the best treatment for each patient. In this endeavour certain glycosylated markers have proven useful, such as the carcinoembryonic antigen (CEA) and the sialyl-Lea antigen (CA 19-9), whose combined preoperative serum evaluation has been considered of interest for the management of CRC (1).
Glycosylation is a key factor modulating the structure and biological functions of glycoproteins. The importance of glycosylation is manifest a) in the fact that 50% of human proteins are glycosylated (2); and b) that about 1% of the genome of prokaryotes and eukaryotes is dedicated to encoding the highly conserved glycosylation enzymes (glycosidases and glycosyltransferases) (3).
Glycoproteins can achieve unique functional properties through certain specific alterations in the terminal composition of their glycan antennae. The fucose content of N-and mucin-type O-glycoproteins, for example, is strictly regulated during ontogeny and cell differentiation (4,5), and similarly, aberrations in the fucosylation of secreted and cell surface proteins are among the most common oligosaccharide modifications during malignant transformation (6,7). Recent evidence suggests that all these changes are not an epiphenomenon in the origin, growth and spread of cancer. Rather, fucose content is currently considered a pathophysiological effector of the malignant phenotype in gastrointestinal (8) and breast cancer (9), among others. This dynamic complexity (10) is played by the crucial role of 13 regulated and organ-specific human fucosyltransferases (FT) known.
FT are essential for the formation of the histo-blood ABH and Lewis antigens, by adding L-fucose residues to the non-reducing terminal oligosaccharides of N-/O-glycoconjugates. Thus located at the distal end of the glycan chain, fucose is exposed to the microenvironment and is therefore expected to account for a part of their distinctive properties. The biosynthesis of Lewis determinants precedes from four main precursors, designated core type 1, 2, 3 and 4 (11). Type 1 and 2 give rise mainly to the H1/H2 antigens, as well as to the monofucosylated (Lea, Lex) and difucosylated (Leb, Ley) Lewis antigens (Fig. 1), all of them part of the N-/O-glycoproteins and glycolipids of the lactoseries. The terminal steps in their formation are due to the sequential action of α(1,2)-, α(1,3)-and α(1,3/4)FT on core 1 and core 2 precursors (Fig. 1). The two known human α(1,2)FT (EC 2.4.1.69) responsible for the initial synthesis of H blood-group antigens (H1 and H2) are encoded by the FUT1 gene (H transferase) and FUT2 gene (Se transferase). Additionally, the FUT4 to FUT7 and FUT9 genes encode FT showing the α(1,4)FT or α(1,3)FT activity (EC 2.4.1.-) required to add a(1,4)-or α(1,3)Fuc to GlcNAc residues and obtain the core 1-derived (Lea/Leb) and core 2-derived (Lex/Ley) antigens, respectively (10,11). However, FUT3 encodes the α(1,3/4)FT Lewis enzyme (EC 2.4.1.65), which exhibits both α(1,3)-and α(1,4)FT activities, and can use core 1/2 (12) and monofucosylated Lea/Lex (13) as substrates.
The colorectal expression of mono-(Lea/Lex) and difucosylated (Leb/Ley) Lewis antigens changes with time according to ontogeny, maturation and different anatomic regions of the normal colorectum (14,15). Furthermore, alterations in ABH and Lewis histo-blood group-related antigen expression are a characteristic feature of carcinomas and have been implicated in different types of intercellular interactions, such as immunological recognition and evasion, apoptosis, adhesion and invasion (11). In this context, basal levels of fucosylation in healthy colorectal tissue are relatively low, but undergo a marked increase during carcinogenesis. There is now clear evidence of neosynthesis and/or overexpression of certain ABH and Lewis antigens in the majority of cancer cells derived from colorectal epithelium (8,14,16). These findings indicate that fucosylated epitopes are typical oncofetal antigens in colorectal cancer, several of them associated with poor clinical prognosis (14-20), and useful as possible CRC biomarkers (7,21-23). This demonstrates the usefulness of molecular biology in clinical setting and highlights the importance of translational efforts to overcome the gap between basic research and clinical practice.
In sum, a) changes in H and Lewis antigen expression in colorectal carcinogenesis have been documented; b) the synthesis of these fucosylated determinants requires several tissue-specific FT acting on core 1 and core 2 precursors; and c) the kinetic alterations of some of these enzymes in CRC have recently been reported by our laboratory (13). Despite this background, contradictory data remain, and new insights in this field are required. Accordingly, we performed a screen of the synthesis and expression of mono-and difucosylated Lewis antigens, a) addressing the enzyme activity of terminal α(1,2), α(1,3) and α(1,4)fucosylation steps from core 1 and 2; and b) assessing the histological expression of core 1-derived (Lea and Leb) and core 2-derived (Lex and Ley) Lewis determinants. Our intention was to characterize the sequential fucosylation of core 1 and core 2 structures in healthy vs. tumour CRC tissue, in order to obtain a general view of the functional status of their biosynthetic pathways. Our findings demonstrated that Ley hyperfucosylation is important at an early stage of colorectal carcinogenesis, and thus the currently available knowledge about the molecular modulation undergone by this family of fucosylated epitopes in CRC has improved.
Materials and methods
Reagents
GDP-L-[14C]-fucose (specific activity 10 GBq/mmol, 270 mCi/mmol) and the scintillation counting mixture Ecoscint H were supplied by New England Nuclear (Ma, USA) and National Diagnostics (Ga, USA), respectively. The oligosaccharides used as exogenous acceptors, GDP-fucose, bicinchoninic acid (BCA), bovine serum albumin (BSA), Dowex 2X8-400, detergents, salts and buffers, were purchased from Sigma Chemical Co. (Mo, USA). The mouse anti-human Lea, Leb, Lex and Ley monoclonal antibodies, as well as the goat anti-mouse, labelled polymer, HRP, EnVisionTM Detection System, were from DAKO (Ca, USA). All other reagents were of analytical quality.
Colorectal specimens and preparation of tissue extracts
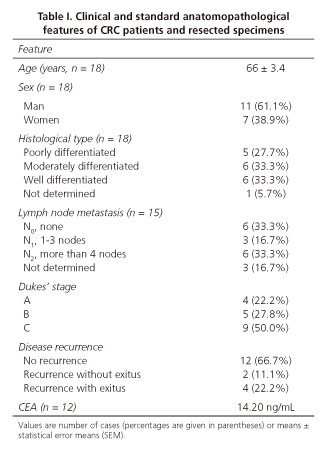
Colorectal tissue specimens were obtained from patients with CRC undergoing surgery at the University Hospital Complex of Ourense (CHUO, Spain) after receiving approval from the appropriate local Institutional Board. After resection, 18 specimens of 1-14 cm obtained from primary colorectal carcinoma and healthy tissue (at least 10 cm distant from the tumour and without any histological trace of malignancy), were washed with ice-cold saline buffer and stored at -80 oC until use. All relevant information, including clinical and anatomopathological features of patients and specimens (Table I), was provided by the Anatomopathological Service of CHUO, following an anonymous and strictly confidential schedule. The histopathological grading of tumours was established according to Dukes' stages (24) and the nodal metastasis (N factor) of the TNM classification (25).
Total cell membranes were obtained as previously described (13). The protein concentration of the enzyme preparations was determined by means of the BCA protein assay, using BSA as the standard.
Fucosyltransferase assays
The standard cocktail for FT reactions contained the following reactants and final concentrations in a total volume of 50 µL: 50 mM Tris-HCl (pH 7.2), 10 mM MnCl2, 3% (v/v) Triton X-100, 100 µM GDP-L-[14C]-fucose/GDP-L-fucose (isotopic dilution 1/200), 0.5 mM acceptor substrate (see later) and 60-100 µg of protein solution. The assays (run in duplicate) were started by adding the donor substrate GDP-L-[14C]-fucose/GDP-L-fucose, and incubated for 90 min at 37 oC in a shaken water-bath. The reactions were stopped with 0.4 mL of cold water and immersion in an ice bath. The incubation of tumour samples and their respective controls of healthy mucosa was always conducted in parallel.
Soluble oligosaccharides corresponding to the non-reducing end of the in vivo fucosylated sequences, or alternative commercial structural analogues, were employed as exogenous acceptor substrates. As some of these oligosaccharides could act as an acceptor substrate for several FT, and vice versa, we chose a set (Table II) covering both the core 1 and 2-derived Lewis biosynthetic pathways.
The reaction products were separated from the isotopic mixture of GDP-L-fucose by ion-exchange chromatography in 3-mL Dowex 2x8-400 columns, and eluted with water in a manifold station (Waters, USA) coupled to a vacuum pump (Millipore, Spain). The eluted fucosylated oligosaccharides were collected in fractions of 0.4 mL and mixed with 5 mL of scintillation counting mixture (Ecoscint H). Radioactivity was measured on a Wallac 1409-12 Scintillator system (Boston, USA). Control assays without exogenous acceptors were carried out to evaluate endogenous non-specific radiolabelling. The enzyme activity was expressed as pmol of GDP-L-fucose transferred per min and mg of protein (µU/mg).
Immunohistochemical assays
Histological slides of tumour and healthy specimens from 18 CRC-resected patients included in the study were fixed in formalin and embedded in paraffin. Sections (4 μm) from selected tissue blocks were deparaffinised in xylene, hydrated in a graded ethanol series, and heated (microwave oven for 20 min) in 0.1 M citric acid buffer (pH 6.0) to unmask the epitopes. The endogenous peroxidase activity was eliminated with 0.5% (v/v) hydrogen peroxide in methanol for 20 min. Likewise, non-specific binding was blocked with 2% BSA for 20 min. Each incubation step was followed by thorough washing of the tissue slides in PBS.
Immunohistochemical staining began with the incubation of the sections (room temperature, 30 min) with primary monoclonal antibodies against Lea (mouse anti-human Lea, isotype IgG1, dilution 1:1000), Leb (mouse anti-human Leb, isotype IgG1, dilution 1:1000), Lex (CD15, mouse anti-human granulocytes, C3D-1 clone, dilution 1:50) and Ley (mouse anti-human Ley, BM-1 clone, dilution 1:1000). After a rinse with PBS, the sections were incubated (room temperature, 30 min) with the secondary antibody bound to peroxidase (goat anti-mouse, labelled polymer, HRP, EnVisionTM Detection System). The peroxidase reaction was visualized by incubating with DAB (3,3'-diaminobenzidine). Finally, after a wash with water, the sections were counterstained with Papanicolau's haematoxylin, dehydrated in a graded ethanol series, washed in xylene and mounted in Dpex mounting medium. Negative controls were performed using PBS instead of primary antibody.
Immunohistochemical staining, detected as a brown precipitate, was evaluated by two expert pathologists belonging to the hospital partner (CHUO), who reached a final conclusion by consensus. The semiquantitative scores of antigen expression were as follows: negative (0), specimen without staining; weak (1), less than 10% of the tissue stained; moderate (2), 10-50% of the tissue stained; high (3), more than 50% of the tissue stained.
Statistical analyses
Statistical analyses were performed using SPSS v. 16.0 for Windows XP. The non-parametric Wilcoxon's test was used for contrasting differences of enzyme activity or antigen expression between control and tumour-paired samples. Correlation between FT activity and antigen expression was carried out by means of Spearman's test, while the comparisons with clinicopathological features were performed with the aid of the χ2 test or Fisher's exact probability test for categorical data (immunohistochemical expression), and the Kruskal-Wallis and the Mann-Whitney U test for continuous data (enzyme activity). The results were considered significant when p < 0.05.
Results
Fucosyltransferase activities involved in the synthesis of core 1-derived (Lea/Leb) and core 2-derived (Lex/Ley) antigens in healthy and tumour tissue from CRC
In the present study, the FT activities involved in the synthesis of Lea/Leb and Lex/Ley antigens (Fig. 1) were evaluated using as acceptors synthetic oligosaccharides that reproduce the terminal non-reducing sequences necessary to complete the terminal steps of the biosynthesis pathway from cores type 1 and type 2 (Table II).
For the screening of the enzymatic synthesis of the core 1 derived antigens, we determined the α(1,2)-and α(1,4)FT activities with lacto-N-biose 1 as the acceptor substrate in healthy and tumour specimens from 12 CRC patients. The results obtained (Table III) showed a statistically significant increase (p < 0.005, according to Wilcoxon's test) in α(1,2)-and α(1,4)FT activity in tumour tissue (186 ± 35.1 μU/mg) compared to healthy tissue (65.4 ± 19 μU/mg). Similarly, the α(1,4)FT activity was assayed with lacto-N-fucopentaose 1 in healthy and tumour specimens from 15 CRC patients (Table III). The enzyme activity in tumour tissue was significantly higher than in healthy tissue (125.4 ± 20.7 μU/mg vs. 64.9 ± 11.9 μU/mg; p < 0.005, according to Wilcoxon's test). The last step of the Leb biosynthetic pathway was tested using Lea oligosaccharide as the acceptor substrate. In this case, the α(1,2)FT activity was measured in healthy and tumour specimens from 8 CRC patients. The results (Table III) showed a statistically significant enhancement of specific activity in tumour tissue in comparison with its healthy counterpart (130.5 ± 15.6 μU/mg vs. 56.2 ± 7.2 μU/mg; p < 0.001, according to Wilcoxon's test).
In the case of enzymes involved in the synthesis of the core 2 derived antigens, α(1,2)FT and α(1,3)FT activities were evaluated together with N-acetyllactosamine as the acceptor substrate. The results of 18 tested CRC patients (Table III) showed a strong increase in enzyme activity in the tumour tissue (108.1 ± 18.9 μU/mg) with respect to the healthy one (53.4 ± 12.2 μU/mg). We analyzed the significance of this increase and found that the differences between tumour and healthy tissues were statistically significant (p < 0.001, according to Wilcoxon's test). The α(1,3)FT enzyme activity involved in the synthesis of Ley antigen was determined using the H2 antigen (2'-fucosyl-N-acetyllactosamine, 2'-FNA) and two structural analogues (2'-fucosyllactose, 2'-FL; and 2'-methyllactose, 2'-ML) as acceptors in 14, 16 and 17 assays, respectively. As shown in table III, the α(1,3)FT activity in tumour tissue increased when employing any of these three chosen acceptors: 126.4 ± 22.9 μU/mg vs. 61.3 ± 10.7 μU/mg for 2'-FNA; 143.6 ± 28.9 μU/mg vs. 38.9 ± 10.9 μU/mg for 2'-FL; and 66.1 ± 8.1 μU/mg vs. 30.9 ± 4.8 μU/mg for 2'-ML. Likewise, in all three cases the reported increase in α(1,3)FT enzyme activity was statistically significant according to Wilcoxon's test (p < 0.001 for 2'-FNA and 2'-FL, and p < 0.005 for 2'-ML). To conclude the enzymatic study of Ley biosynthesis, we assayed the direct fucosylation of the Lex and its structural analogue 3'-fucosyllactose (3'-FL) (Table III). The α(1,2)FT activity on these two acceptors was increased in the tumour tissue (88.2 ± 14.4 μU/mg for Lex; 70.7 ± 17.2 μU/mg for 3'-FL) with respect to the healthy one (54.3 ± 11.9 μU/mg for Lex; 63.2 ± 29.5 μU/mg for 3'-FL). The enhancement for Lex acceptor was statistically significant (p < 0.001 according to Wilcoxon's test).
Immunohistochemical expression of core 1-derived (Lea/Leb) and core 2-derived (Lex/Ley) antigens in healthy and tumour tissue from CRC
The immunohistochemical expression of Lea/Leb and Lex/Ley antigens was analysed in tumour and healthy-paired specimens from 12/10 and 12/13 CRC-resected patients, respectively. The cell expression of all of them showed up as a brown and granular staining with the same cell and tissue distribution (Fig. 2).
The expression scores (Fig. 3) indicated that healthy mucosa showed a high level of Lea expression (3) in a remarkable number of specimens (58.3%), while the remainder showed moderate (2) (16.7%) or negative (0) expression (25%). Simultaneously, in the tumour tissue specimens the most important changes were as follows: negative staining in 33.3% of cases, weak expression in 8.3% of cases, and a reduction in the percentage of cases with a high level of expression (41.7%). No change in the number of specimens with moderate expression (16.7%) was observed. The study of Leb expression revealed specimens of healthy mucosa showing the antigen with an equal distribution among negative (30%), moderate (30%) and high (40%) expression. Meanwhile, among the specimens of CRC tumour tissue, Leb expression showed the same profile as in healthy tissue, with the exception of a decrease in the number of specimens with negative expression (falling to 20%) and the scoring of weak staining in 10% of them. When these tumour and healthy Lea and Leb percentages of expression were compared using Wilcoxon's test, we found no statistically significant differences.
As for the core 1-derived antigens, the Lex expression in the control tissue distributed in 50% of specimens with weak (1) expression, 25% with moderate (2) expression and 25% with negative (0) expression. In the case of tumour CRC specimens, 66.7% of them showed negative Lex expression, 25% moderate and 8.3% high (3) expression. Wilcoxon's test indicated that these differences of expression were not statistically significant. However, the results of the immunohistochemical study of Ley antigen in 13 CRC patients differed. Negative expression (61.5%) was predominant in healthy specimens (23.1% weak, and 7.7% moderate/high expression), whereas in tumour specimens Ley expression was greatly increased, with 84.6% of 13 patients studied being positive: 7.7% weak intensity, 15.4% moderate and 61.5% of specimens showing high Ley expression. Wilcoxon's test indicated that the increase in Ley expression in tumour tissue was statistically significant (p < 0.01).
Relationship between fucosyltransferase activity or antigen expression and the clinicopathological features of CRC patients and specimens
Having studied the functional activity of the biosynthetic pathways of mono-and difucosylated Lewis antigens in tumour and healthy-paired CRC specimens, as well as their histological expression, we decided to investigate the correlation between these variables and the most important clinicopathological features of CRC patients and specimens (Table I). The results of this statistical study detected no statistical correlation (r < 0.7) between the degree of expression of either Lea/Leb or Lex/Ley antigens in each specimen and the corresponding enzyme activity of the biosynthetic FT involved. Similarly, comparison of antigen expression, as well as their biosynthetic enzyme activities, with the macroscopic and microscopic characteristics of the tumours (anatomopathological stage, degree of differentiation, lymph node metastasis, existence of disease recurrence and post-operatory CEA levels) showed no statistical significance in either case.
Discussion
Initially discovered on red cells, ABH and Lewis-related determinants show a much wider distribution in humans, being mainly present on foetal epithelia and reexpressed in carcinomas (11). Derived from core 1 and 2 precursors, their structural difference lies in the position of the glycosidic bond between Gal and GlcNAc residues in the non-reducing terminals of glucide chains of protein and lipid carriers (Fig. 1). This positional isomerism allows accessibility to fucosylation by different FT, and therefore the products of their action also differ (H1/Lea/Leb from core 1, and H2/Lex/Ley from core 2). The work herein discussed focused on the functional characterization of terminal fucosylation steps of mono-(Lea, Lex) and difucosylated (Leb, Ley) Lewis antigens in healthy and tumour-paired tissue from CRC-resected patients. Additionally, from histological sections of patients undergoing CRC surgical resection (Fig. 2), we evaluated the immunohistochemical expression of these antigens (Fig. 3).
The screening of FT activities necessary for the terminal steps of core 1-derived Lea/Leb and core 2-derived Lex/Ley synthesis showed a generalized activation in tumour tissue of CRC patients. As table III shows, the activity of all FT evaluated resulted increased in a statistically significant way. In this regard, a corresponding enhancement of Lea and/or Leb expression in tumour tissue would be expected. However, the expression levels of these core 1-derived antigens were not modified in relation to the malignant transformation of CRC tissue (Fig. 3), in spite of previous evidence showing a correlation between the increased expression of Leb and the Dukes' stage of each specimen (19,26). On the contrary, other studies have reported the uniform Lea distribution throughout the colon before and after the occurrence of CRC (27,28), or even its reduced expression in parallel with the appearance of colorectal malignancy (16,20). In any case, our apparent increase of α(1,2)FT activity in the tumour colonic tissue is in line with histological (29) and α(1,2)FT cDNA-transfected rat colon cell lines (30) studies, and with them we suggest that since this key enzyme acts on core 1, it could be responsible for regulating the expression of both Lea and Leb antigens, in competition with the α(1,4)FT for this common acceptor (29).
The synthesis of colon Leb (and Ley) antigen is completed by other α(1,2)FT, which directly fucosylates Lea to Leb (and Lex to Ley) (13). The genetic origin of this a(1,2)FT remains uncertain, but all evidence suggests that both activities are associated with the FUT3 gene encoding the α(1,3/4)FT or Lewis enzyme (31,32). The determination of this enzyme activity using Lea as acceptor showed a statistically significant increase in tumour tissue, of the same magnitude as the abovementioned FT activities (Table III). It has been reported that increased cellular Lea fucosylation reduces Lea expression in favour of Leb (29). Conversely, in 8 patients in our cohort we found coexpression of both antigens in the healthy and/or tumour tissue from CRC patients, thus indicating that not all the pool of Lea epitope was transformed into Leb, and that the direct fucosylation of Lea to Leb is not a specific feature of the neoplastic status, as previously suggested (33,34).
The results of this study are consistent with the activation of FT responsible for the biosynthesis of Lea and Leb antigens. This fact is relevant, since to date no functional characterization of the terminal steps of their biosynthetic pathway has been undertaken. However, this activation is not the only variable that controls the production of the end products of the pathway -the activity of upstream acting enzymes should also be considered. In the synthesis of gangliosides in melanoma and neuroblastoma, for example, there is competition between sialyltransferases and FT for the same substrates (35). Similarly, our observations in CRC tumour tissue are consistent with the enhancement of core 1 sialylation and channelling of the corresponding associated antigens (i.e. sialyl-Lea), (36), to the detriment of their fucosylated counterparts studied herein. So, core 1 would be channelled into sialyl-Lec, which would deprive colorectal mucosa of both the direct precursor of Lea (core 1) and the intermediary to render Leb (H1). In line with this hypothesis, it has been reported that the enhancement of α(2,3)sialyltransferases and α(1,3/4)FT is one of the principal mechanisms leading to increased sialyl-Lea (and sialyl-Lex) expression in different neoplasias (37).
Regarding the core 2-derived Lex/Ley antigens, it is reasonable to propose that activated FT activities (Table III) led to the up-regulation of Ley at the expense of decreased Lex (Fig. 3). The overexpression of Ley may indicate that the predominant activity is that of α(1,2)FT (Fig. 1). This possibility is supported by previous evidence giving this enzyme (H transferase) a key regulatory role in the up-regulation of H2 and Ley antigens in tumour tissue of CRC patients (38). Moreover, the assay of α(1,3)FT responsible for the synthesis of Ley showed the existence of a statistically significant increase in H2 fucosylation, thus rendering the Ley antigen, as previously reported (39). This was corroborated by the negative expression found in control and tumour tissue of four CRC patients tested for H2. As pointed out with antigens derived from core 1, the biosynthesis of antigens derived from core 2 includes the transformation of Lex into Ley by the action of α(1,2)FT Lewis enzyme. The statistically significant increase of this α(1,2)FT activity in tumour tissue of CRC, along with the reduction in Lex expression and the increase in Ley, suggest that this enzyme may be partially responsible for the Ley overexpression reported by us and others, and that the enhancement of this FT activity may be closely linked to colorectal carcinogenesis (33).
Several authors have found no significant differences in the expression of Ley in CRC tumour tissue in comparison with healthy mucosa (40). However, our reported change in Ley expression is consistent with those of previous studies (27,28,41,42) which have justified the classical consideration of this determinant as an oncofetal antigen (43) and a premalignant biomarker of distal CRC (18,40). In this regard, it should be noted that tumour biomarkers are not only monitors for diagnosis or monitoring of patients, but represent real characteristics of cancer cells. Thus, the mechanisms leading to the expression of any tumour marker should be disclosed, since this knowledge will accelerate and make more plausible its translation to clinical therapy.
Taken as a whole, the results of this study confirm the activation of the biosynthetic pathways of mono-and difucosylated Lewis histo-blood antigens in tumour tissue of CRC-resected patients. The kinetic activation of core-2 derived pathway allows us to hypothesize that increased activity on N-acetyllactosamine (core 2) would essentially result from the overactivity of tumour α(1,2)FT (H transferase, 38). Consequently, the activation of this enzyme stimulated the enhanced expression of oncofetal H2 epitope (44), and the overproduction of H2 activated the α(1,3)FT enzyme (this enzyme acts on H2 in preference to core 2 as a substrate) leading to the overexpression of Ley antigen, probably at the expense of Lex (and H2) determinants. In other words, the malignant transformation of the colon causes positive modulation of the biosynthesis pathway of Lewis antigens derived from core 2, which promotes the production of Ley instead of Lex. The functional significance of Ley (and Leb) up-regulation has been linked to the stimulation of cancer propagation and metastasis, since this epitope is expressed by adhesive and cell motility glycoproteins (45), and has been found in glycan homophilic interactions involved in the aggregation of tumour cells or in their adhesion to the vascular endothelium (4). In addition, a role for Ley (Leb and others) in the resistance of epithelial cells to apoptosis has been suggested (11). Based on this evidence, it is not surprising that treatment with anti-Ley antibodies has been found to slow down or even cause regression of colon tumours in animal models (46).
The cohort included in this study was large enough to confirm the activation of the biosynthetic Lea/Leb and Lex/Ley pathways, as well as the Ley overexpression, but not to find significant correlations with the standard clinicopathological features of specimens and patients. The inconsistency between the expression of Lewis antigens and the specific activity of FT involved in their synthesis, as well as their discrepancies with clinicopathological features, could be explained by the low size of the cohort and the variation among individuals. Also, we must keep in mind that scores of antigen expression derived from an immunohistochemical semiquantitative evaluation, while the values of enzyme activities allowed a quantitative biochemical determination. In this sense, it will be necessary to undertake larger retrospective studies to elucidate the relationship between enhanced biosynthetic enzymes and/or Ley hyperexpression and progression of the neoplasia and the prognosis of CRC patients.
Acknowledgments
This research would not have been possible without the collaboration and technical assistance of the Pathology Service of the University Hospital Complex of Ourense (CHUO, Ourense, Spain).
References
1. Chen CC, Yang SH, Lin JK, et al. Is it reasonable to add preoperative serum level of CEA and CA 19-9 to staging for colorectal cancer? J Surg Res 2005;124:169-74. [ Links ]
2. Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS. PROT database. Biochim Biophys Acta 1999;1473:4-8. DOI: 10.1016/S0304-4165(99)00165-8. [ Links ]
3. Coutinho PM, Deleury E, Davies GJ, et al. An evolving hierarchical family classification for glycosyltransferases. J Mol Biol 2003;328:307-17. DOI: 10.1016/S0022-2836(03)00307-3. [ Links ]
4. Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo (glyco) lipid metabolism. Cancer Res 1996;56:5309-18. [ Links ]
5. Staudacher E, Altmann F, Wilson IBH, et al. Fucose in N-glycans: From plant to man. Biochim Biophys Acta 1999;1473:216-36. DOI: 10.1016/S0304-4165(99)00181-6. [ Links ]
6. Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem 2008;143:725-9. DOI: 10.1093/jb/mvn011. [ Links ]
7. Miyoshi E, Moriwaki K, Terao N, et al. Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules 2012;2:34-45. DOI: 10.3390/biom2010034. [ Links ]
8. Moriwaki K, Miyoshi E. Fucosylation and gastrointestinal cancer. World J Hepatol 2010;2:151-61. DOI: 10.4254/wjh.v2.i4.151. [ Links ]
9. Listinsky JJ, Siegal JP, Listinsky CM. The emerging importance of α-L-fucose in human breast cancer: A review. Am J Transl Res 2011;3:292-322. [ Links ]
10. Becker DJ, Lowe JB. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003;13:41R-53R. DOI: 10.1093/glycob/cwg054. [ Links ]
11. Le Pendu J, Marionneau S, Cailleau-Thomas A, et al. ABH and Lewis histo-blood group antigens in cancer. APMIS 2001;109:9-31. DOI: 10.1111/j.1600-0463.2001.tb00011.x. [ Links ]
12. Kokowska-Latallo JF, Larsen RD, Nair RP, et al. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4) fucosyltransferase. Genes Dev 1990;4:1288-303. DOI: 10.1101/gad.4.8.1288. [ Links ]
13. Muinelo-Romay L, Gil-Martín E, Fernández-Briera A. α(1,2)fucosylation in human colorectal carcinoma. Oncol Lett 2010;1:361-6. [ Links ]
14. Kim YS, Yuan M, Itzkowitz SH, et al. Expression of Ley and extended Ley blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res 1986;46:5985-92. [ Links ]
15. Wolf BC, Salem RR, Sears HF, et al. The expression of colorectal carcinoma-associated antigens in the normal colonic mucosa. An immunohistochemical analysis of regional distribution. Am J Pathol 1989;135:111-9. [ Links ]
16. Schoentag R, Primus FJ, Kuhns W. ABH and Lewis blood group expression in colorectal carcinoma. Cancer Res 1987;47:1695-700. [ Links ]
17. Holmes EH, Ostrander GK, Clausen H, et al. Oncofetal expression of Lex carbohydrate antigens in human colonic adenocarcinomas. J Biol Chem 1987;262:11331-8. [ Links ]
18. Kim YS, Itzkowitz SH. Carbohydrate antigen expression in adenoma-carcinoma sequence. Prog Clin Biol Res 1988;279:241-50. [ Links ]
19. Naitoh H, Nakajima T, Nagamachi Y, et al. A clinicopathological evaluation of anti fucosylated antigen antibody (YB-2) in colorectal carcinoma. Glycosylation Dis 1994;1:31-6. DOI: 10.1007/BF00917466. [ Links ]
20. Nakagoe T, Fukushima K, Hirota M, et al. An immunohistochemical employer of monoclonal antibodies against Le(a), sialyl Le(a), Le(x), and sialyl Le(x) antigens in primary colorectal, carcinomas and lymph node and hepatic lesions. J Gastroenterol 1994;29:129-38. DOI: 10.1007/BF02358673. [ Links ]
21. Blaszczyk M, Pak KY, Herlyn M, et al. Characterization of Lewis antigens in normal colon and gastrointestinal adenocarcinomas. Proc Natl Acad Sci 1985;82:3552-6. DOI: 10.1073/pnas.82.11. 3552. [ Links ]
22. Muramatsu T. Carbohydrate signals in metastasis and prognosis of human carcinomas. Glycobiology 1993;3:294-6. DOI: 10.1093/glycob/3.4.291. [ Links ]
23. Park SY, Lee SH, Kawasaki N, et al. alpha1-3/4 fucosylation at Asn 241 of Beta-haptoglobin is a novel marker for colon cancer: A combinatorial approach for development of glycan biomarkers. Int J Cancer 2011;130:2366-76. DOI: 10.1002/ijc.26288. [ Links ]
24. Dukes CE. The classification of cancer of the rectum. J Pathol Bacteriol 1932;35:323-32. DOI: 10.1002/path.1700350303. [ Links ]
25. Sobin H, Wittekind CH. TNM classification of malignant tumours. 6th ed. New York: Wiley-Liss; 2002. p. 72-6. [ Links ]
26. Ernst CS, Shen JW, Litwin S, et al. Multiparameter evaluation of the expression in situ of normal and tumor-associated antigens in human colorectal carcinoma. J Natl Cancer Inst 1986;77:387-95. [ Links ]
27. Sakamoto J, Furukawa K, Gordon-Cardo C, et al. Expression of Lewisa, Lewisb, X and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell lines. Cancer Res 1986;46:1553-61. [ Links ]
28. Blasco E, Torrado J, Cosme A, et al. Expression of Lewis antigenic determinants in colorectal adenocarcinomas. J Exp Cell Biol 1989;57:153-8. DOI: 10.1159/000163519. [ Links ]
29. Ørntoft T, Holmes EH, Johnson P, et al. Differential tissue expression of the Lewis blood group antigens: enzymatic, immunohistologic, and immunohistochemical evidence for Lewis a and b antigen expression in Le(a-b-) individuals. Blood 1991;77:1389-96. [ Links ]
30. Goupille C, Marionneau S, Bureau V, et al. α(1,2) fucosyltransferase increases resistance to apoptosis of rat colon carcinoma cells. Glycobiology 2000;10:375-82. DOI: 10.1093/glycob/10.4.375. [ Links ]
31. Chandrasekaran EV, Jain RK, Rhodes JM, et al. Expression of blood group Lewis b determinant from Lewis a: Association of this novel α(1,2)-L-fucosylating activity with the Lewis type α(1,3/4)-L-fucosyltransferase. Biochemistry 1995; 34: 4748-56. DOI: 10.1021/bi00014a032. [ Links ]
32. Nakamura JI, Mogi A, Asao T, et al. Evidence that the aberrant α(1,2) fucosyltransferase found in colorectal carcinoma may be encoded by FUT III (Le gene). Anticancer Res 1997;17:4563-9. [ Links ]
33. Yazawa S, Nakamura J, Asao T, et al. Aberrant α1α2fucosyltransferase found in human colorectal carcinoma involved in the accumulation of Leb and Y antigens in colorectal tumors. Jpn J Cancer Res 1993;84:989-95. DOI: 10.1111/j.1349-7006.1993.tb00190.x. [ Links ]
34. Yazawa S, Nishimura T, Ide M, et al. Tumor-related expression of α1,2fucosylated antigens on colorectal carcinoma cells and its suppression by cell-mediated priming using sugar acceptors for α1,2fucosyltransferase. Glycobiology 2002;12:545-53. DOI: 10.1093/glycob/cwf070. [ Links ]
35. Ruan S, Lloyd KO. Glycosylation pathways in the biosynthesis of gangliosides in melanoma and neuroblastoma cells: Relative glyco-syltransferases levels determine ganglioside patterns. Cancer Res 1992;70:1467-76. [ Links ]
36. Villar-Portela S, Vázquez-Martín C, Muinelo-Romay L, et al. sLea and sLex expression in colorectal cancer: implications for tumourigenesis and disease prognosis. Histol Histopathol 2011;26:1305-16. [ Links ]
37. Kannagi R, Izawa M, Koike T, et al. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 2004;95:377-84. DOI: 10.1111/j.1349-7006.2004.tb03219.x. [ Links ]
38. Sun J, Thurin J, Cooper HS, et al. Elevated expression of H-type GDP-L-fucose:α-D-galactoside α-2-L-fucosyltransferase is associated with human colon-adenocarcinoma progression. Proc Natl Acad Sci 1995;92:5724-8. DOI: 10.1073/pnas.92.12.5724. [ Links ]
39. Yazawa S, Madiyalakan R, Izawa H, et al. Cancer-associated elevation of α(1,3) activity in human serum. Cancer 1988;62:516-20. DOI: 10.1002/1097-0142(19880801)62:3<516::AID-CNCR2820620313> 3.0.CO;2-4. [ Links ]
40. Nakagoe T, Fukushima K, Hirota M, et al. An immunohistochemical study of the distribution of blood group substances and related antigens in primary colorectal carcinomas and metastatic lymph node and liver lesions, using antibodies against A, B, H type 2, Le(a), and Le(x) antigens. J Gastroenterol 1994;29:265-75. DOI: 10.1007/BF02358364. [ Links ]
41. Cordon-Cardo C, Lloyd K.O, Sakamoto J, et al. Immunohistologic expression of blood-group antigens in normal human gastrointestinal tract and colonic carcinoma. Int J Cancer 1986;37:667-76. DOI: 10.1002/ijc.2910370505. [ Links ]
42. Bara J, Mollicone R, Herreo-Zabaleta E, et al. Ectopic expression of the Y (Ley) antigen defined by monoclonal antibody 12-4LE in distal colonic adenocarcinomas. Int J Cancer 1988;41:683-9. DOI: 10.1002/ijc.2910410508. [ Links ]
43. Hakomori S, Nudelman E, Levery SB, et al. Novel fucolipids accumulating in human adenocarcinoma. I. Glycolipids with di-or tri-fucosylated type 2 chain. J Biol Chem 1984;259:4672-80. [ Links ]
44. Cooper HS, Malecha MJ, Bass C, et al. Expression of blood group antigens H-2, Ley, and sialylated-Lea in human colorectal carcinoma. Am J Pathol 1991;138:103-10. [ Links ]
45. Prokopishyn NL, Puzon-McLaughlin W, Takada Y, et al. Integrin alpha3beta1 expressed by human colon cancer cells is a major carrier of oncodevelopmental carbohydrate epitopes. J Cell Biochem 1999;72:189-209. DOI: 10.1002/(SICI)1097-4644(19990201) 72:2<189::AID-JCB4>3.0.CO;2-N. [ Links ]
46. Trail PA, Willner D, Bianchi AB, et al. Enhanced antitumor activity of paclitaxel in combination with the anticarcinoma immunoconjugate BR96-doxorubicin. Clin Cancer Res 1999;5:3632-8. [ Links ]
![]() Correspondence:
Correspondence:
Emilio Gil Martín.
Department of Biochemistry,
Genetics and Immunology.
Faculty of Biology.
University of Vigo.
Campus Lagoas-Marcosende.
36310 Vigo, Spain
e-mail: egil@uvigo.es
Received: 26-03-2015
Accepted: 09-06-2015