My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.11 Madrid Nov. 2015
Neomycin and bacitracin reduce the intestinal permeability in mice and increase the expression of some tight-junction proteins
La neomicina y bacitracina disminuyen la permeabilidad intestinal en ratones e incrementan la expresión de algunas proteínas de unión estrecha
Rebeca Nevado1, Raquel Forcén1, Elena Layunta1, María Divina Murillo1,2 and Laura Grasa1,2,3
1 Departamento de Farmacología y Fisiología. Facultad de Veterinaria. Universidad de Zaragoza. Zaragoza, Spain.
2 Instituto de Investigación Sanitaria de Aragón (IIS).
3 Instituto Agroalimentario de Aragón (IA2) (Universidad de Zaragoza-CITA). Zaragoza, Spain
This work was funded by Gobierno de Aragón (B61/2014) and Universidad de Zaragoza (JIUZ-2013-BIO-08), Spain.
ABSTRACT
Background: Tight-junction (TJ) proteins regulate paracellular permeability. Gut permeability can be modulated by commensal microbiota. Manipulation of the gut microbiota with antibiotics like bacitracin and neomycin turned out to be useful for the treatment of diarrhoea induced by Clostridium difficile or chemotherapy drugs.
Aim: To evaluate the effects of the microbiota depletion evoked by the oral administration of neomycin and bacitracin on the intestinal permeability and expression of TJ proteins in mice.
Methods: Mice received neomycin and bacitracin orally for 7 days. Intestinal permeability was measured by the fluorescein-isothiocyanate-dextran (FITC-dextran) method. The gene expression of TJ proteins in the intestine was determined by real time-PCR.
Results: FITC-dextran levels in serum were reduced by half in antibiotic-treated mice, indicating a reduction of intestinal permeability. Antibiotics increased the expression of zonula occludens 1 (ZO-1), junctional adhesion molecule A (JAM-A, and occludin in the ileum and ZO-1, claudin-3, and claudin-4 in the colon.
Conclusion: The combination of neomycin and bacitracin reduce intestinal permeability and increase the gene expression of ZO-1, junctional adhesion molecule A (JAM-A), and occludin in the ileum and ZO-1, claudin-3, and claudin-4 in the colon.
Key words: Antibiotics. Microbiota. Tight-junction proteins. Intestinal permeability.
RESUMEN
Antecedentes: las proteínas de unión estrecha (UE) regulan la permeabilidad paracelular. La permeabilidad intestinal puede estar modulada por la microbiota comensal. Las manipulaciones de la microbiota intestinal con antibióticos como la bacitracina y neomicina han resultado ser útiles para el tratamiento de la diarrea inducida por Clostridium difficile o los fármacos quimioterápicos.
Objetivos: evaluar los efectos de la depleción de la microbiota mediante la administración oral de bacitracina y neomicina sobre la permeabilidad intestinal y la expresión de las proteínas de UE en ratón.
Métodos: los ratones recibieron por vía oral la combinación de neomicina y bacitracina durante 7 días. La permeabilidad intestinal se cuantificó con el método del dextrano marcado con isotiocianato de fluoresceína (FITC-dextrano). La expresión de las proteínas de UE en el intestino se determinó mediante PCR a tiempo real.
Resultados: los niveles de FITC-dextrano en suero se redujeron a la mitad en los ratones tratados con antibióticos, indicando una reducción de la permeabilidad intestinal. Los antibióticos incrementaron la expresión de zónula occludens 1 (ZO-1), molécula de adhesión de unión A (JAM-A) y ocludina en íleon y de ZO-1, claudina-3 y claudina-4 en colon.
Conclusiones: la combinación de neomicina y bacitracina reduce la permeabilidad intestinal e incrementa la expresión de ZO-1, JAM-A y ocludina en íleon y ZO-1, claudina-3 y claudina-4 en colon.
Palabras clave: Antibióticos. Microbiota. Proteínas de unión estrecha. Permeabilidad intestinal.
Introduction
The intestinal epithelium provides a selective, permeable barrier achieved by the presence of intercellular tight-junction (TJ) structures, which regulate paracellular permeability. The TJ protein complex consists of transmembrane and intracellular scaffold proteins. Four transmembrane proteins, occludin, claudins (24 members), junctional adhesion molecule (JAM), and tricellulin, have been identified. These transmembrane proteins interact with cytosolic scaffold proteins such as zonula occludens (ZO) proteins (1).
Intestinal epithelial cells are continuously interacting with an extensive intestinal microbiota, which modulate the intestinal permeability directly through the release of toxins, cellular structural components, or metabolites, or indirectly through its effects on host immune cells (2).
Antibiotic intake for the treatment of various diseases of bacterial origin obviously produces an alteration of the intestinal microbiota. This alteration of the intestinal microbiota can have beneficial effects and contribute to the restoration of intestinal homeostasis. Bacitracin has been used orally for the treatment of Clostridium difficile-associated diarrhoea and colitis (3,4). Oral neomycin is indicated for suppression of intestinal bacterial flora in patients undergoing colorectal surgery (5,6) and as a means of decreasing colonic bacteria and the production of ammonia in hepatic encephalopathy (7). Recently, it has been reported that the oral administration of neomycin plus bacitracin prevents diarrhoea induced by chemotherapy drugs used in the treatment of advanced colorectal cancer (8). However, prolonged use of antibiotics has also been described as a factor that, by altering the intestinal microbiota, can contribute to the development and/or maintenance of chronic intestinal diseases such as inflammatory bowel disease or irritable bowel syndrome, pathologies with altered intestinal permeability (9).
The aim of the present study is to evaluate the effects of the microbiota depletion induced by the oral administration of neomycin and bacitracin on intestinal permeability and the expression of certain TJ proteins in mice.
Methods
Animals and treatment with antibiotics
All procedures were approved by the Ethics Committee for Animal Experiments from the University of Zaragoza, Spain (Project Licence PI36/12).
Female C57BL/10 mice (5 to 7 weeks old) received a combination of non-absorbable antibiotics (neomycin 20 mg and bacitracin 20 mg per mouse, AppliChem, Barcelona, Spain) by oral gavage (0.2 mL) for 7 consecutive days. Bacitracin and neomycin were diluted in sterile, deionized water, and the pH of the solution was adjusted to 4.0 to prevent inactivation of bacitracin. Pimaricin (5 µg per mouse) was added to the antibiotic solution to prevent yeast overgrowth. Control mice received sterile, deionized water (0.2 mL). This combination of antibiotics has been used previously by our group to induce significant depletion in commensal microbiota (10).
We used 2 groups of 8 mice (control and treated) to assess intestinal permeability in vivo and 2 groups of 8 mice (control and treated) to study the gene expression of the TJ proteins.
Intestinal permeability in vivo
After a 14 h fast, mice were gavaged with 60 mg per 100 g body weight of fluorescein-isothiocyanate-dextran (FITC-dextran, FD4, 3.000-5.000 kD, Sigma-Aldrich, Madrid, Spain) in a volume of 0.2 mL. Blood samples were obtained by cardiac puncture at 4 h after administration of FITC-dextran, as it has been described that dextran levels in serum are at maximum at this point (11). The blood was centrifuged at 3,000 rpm for 5 min at room temperature to obtain the serum. Fluorescence intensity of each serum sample (DTX 880 Multimode Detector, Beckman Coulter, CA, USA) was measured, and the concentrations of FITC-dextran were determined from standard curves generated by the serial dilution of FITC-dextran. Results were expressed as ng FITC-dextran mL-1 serum per 100 g body weight (bw).
Study of mRNA expression of TJ proteins
The expression of zonula occludens 1 (ZO-1), junctional adhesion molecule A (JAM-A), occludin, and claudin-3, -4, and -7 mRNA was quantified by real time-PCR. Ileum (i.e. the last 6 cm of the small intestine) and proximal colon samples from control and antibiotic-treated mice were extracted and preserved in RNAlater solution (Ambion, Life Technologies, Madrid, Spain) at 4 oC overnight. The samples were kept at -80 oC until the RNA extraction with the RNeasy mini Kit (Qiagen, Hilden, Germany). Complementary DNA (cDNA) was synthesized by reverse transcription using the AffinityScript Multiple Temperature cDNA synthesis Kit (Stratagene, La Jolla, CA, USA), and it was used to determine the expression levels of mRNA of the TJ proteins. Reactions were run using the StepOnePlus Real-Time PCR System (Life Technologies, Carlsband, California, USA). The reaction mixture (10 µL) comprised 4.5 µL FastStart Universal SYBR Green Master (Roche, Mannheim, Germany), 0.5 µL of each primer 30 µM (Table I), 2.5 µL of sterile distilled water, and 2 µL of DNA template (100 ng µL-1). Each sample was run in triplicate, and the mean Ct was determined from the 3 runs. Relative TJ proteins' mRNA expression under each experimental condition (control or treatment) was expressed as ΔCt = Ct TJ protein - Ct calibrator. GAPDH housekeeping gene expression was used as a calibrator after verification of its stability under our experimental conditions. Then, relative TJ proteins' mRNA expression was calculated as ΔΔCt = ΔCt treatment - ΔCt control. Finally, the relative gene expression levels were converted and expressed as fold change respective to the control (=2-ΔΔCt).
Data analysis and statistics
Results were expressed as the mean ± S.E.M. with n denoting the number of animals used. The Mann-Whitney U test was used to compare the data, and differences with P-values < 0.05 were considered to be statistically significant.
Results
Effect of neomycin and bacitracin on intestinal permeability
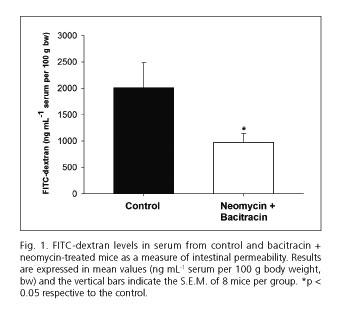
As shown in figure 1, water-drinking mice showed FITC-dextran levels in serum of 2009 ± 473.5 ng mL-1 serum per 100 g of body weight. Mice treated with antibiotics showed a reduction of approximately half in FITC-dextran levels (973.4 ± 172.8 ng mL-1 serum per 100 g of body weight) respective to controls.
Effect of neomycin and bacitracin on mRNA expression of TJ proteins
Figure 2 shows the mRNA expression levels of ZO-1, JAM-A, occludin, and claudins-3, -4, and -7 found in the ileum (Fig. 2A) and colon (Fig. 2B) of mice treated with antibiotics respective to controls. Antibiotic treatment increased the expression of ZO-1, JAM-A, and occludin in the ileum and ZO-1, claudin-3, and claudin-4 in the colon.
Discussion
Previous results by our group have shown that oral administration of neomycin and bacitracin induces a large depletion of the intestinal microbiota, a slight intestinal inflammation, a reduction of faecal output, a slowing of intestinal transit, and a reduction of the contractility in vitro in the ileum and colon in mice (10). In this work, we have evaluated in these same mice treated with antibiotics the permeability of the small intestine and colon to FITC-dextran after 4 h of administration. Other authors have shown that the FITC-dextran can reach the colon at 3 h (12), and the maximum concentrations of FITC-dextran are found in the serum at 4 h after its oral administration (11). Our results indicate that mice treated with neomycin and bacitracin show reduced intestinal permeability to FITC-dextran. However, others have shown that bacitracin increases paracellular uptake of FITC-dextran (4,000 kD) in epithelial cells of human colon carcinoma (Caco-2) (13). Moreover, neomycin increases the absorption of sugars and has direct effects on the morphology of rat intestinal epithelium. However, neomycin does not produce these effects on hamsters, indicating that there are specific differences between species in terms of the susceptibility of the epithelium to neomycin (14). The fact that intestinal transit is slowed down in mice treated with neomycin and bacitracin might explain the lower arrival of FITC-dextran to the intestinal epithelial cells of the ileum and colon and therefore, less paracellular absorption.
On the other hand, there are several examples showing that antibiotic treatment is effective in restoring normal intestinal permeability. Thus, ciprofloxacin and metronidazole improve the impaired mucosal barrier function in mice with cystic fibrosis (15). Oral rifaximin prevents the impairment of intestinal barrier function induced by water-avoidance stress (16).
Among the mechanisms that may modify the intestinal permeability are alterations in TJ proteins' expression, localization, or function (2). In our study, we evaluate the direct effect of the combination of neomycin and bacitracin on gene expression of some TJ proteins. All segments of the small and large intestine of mice show high levels of expression of ZO-1, JAM-A, occludin, and claudins-3, -4, and -7 (17). In our study, the depletion of the microbiota by the administration of neomycin and bacitracin induced an increase in gene expression of ZO-1, JAM-A, and occludin in the ileum and ZO-1, claudin-3, and claudin-4 in the colon. These results indicate that the intestinal microbiota might modulate the transcription of these proteins and, in turn, regulate intestinal permeability. However, it should be noted that changes in gene expression do not always imply a change in functional protein levels and, therefore, a change in the functionality of the TJs.
Our study shows that the microbiota might regulate gene expression of TJ proteins differently in the ileum and colon. Thus, increased expression of ZO-1, JAM-A, and occludin proteins may contribute to the reduced permeability of FITC-dextran observed in the ileum. It has been described that ZO proteins play an important role in regulating the assembly of TJs (1). In vivo and in vitro studies demonstrate that JAM-A participates in the regulation and maintenance of the TJ barrier (1). The JAM-A knockout mice exhibit higher permeability to dextran in the colon compared to wild-type mice (18). Studies in Caco-2 cells and mouse intestines have shown that occludin knockdown induces an increase in paracellular permeability to macromolecules (19).
Conversely, increased expression of ZO-1, claudin-3, and claudin-4 might contribute to reduced permeability to FITC-dextran observed in the colon. Thus, claudin-3 and claudin-4 are involved in barrier formation and decrease the paracellular permeability (1). However, the level of claudin-7 is not altered in the ileum or colon, and this protein is involved in pore formation and increased paracellular permeability (1), which supports our results.
Finally, other antibiotics have been shown to modify the expression of TJ proteins. Oligomycin alleviated the IFN-γ and TNF-α caused morphological redistributions of TJ proteins ZO-1 and occludin (20). Minocycline exerted intestinal anti-inflammatory effects and attenuated the reactivation of colitis by increasing the expression of ZO-1 (21).
In conclusion, the depletion of the microbiota induced by neomycin and bacitracin reduces intestinal permeability and increases expressions of ZO-1, JAM-A, and occludin in the ileum and ZO-1, claudin-3, and claudin-4 in the colon.
References
1. Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013;70:631-59. DOI: 10.1007/s00018-012-1070-x. [ Links ]
2. Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 2012;24:503-12. DOI: 10.1111/j.1365-2982.2012.01921.x. [ Links ]
3. Bricker E, Garg R, Nelson R, et al. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev 2005 CD004610. [ Links ]
4. Nelson R. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev 2007:CD004610. [ Links ]
5. Cannon JA, Altom LK, Deierhoi RJ, et al. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum 2012;55:1160-6. DOI: 10.1097/DCR.0b013e3182684fac. [ Links ]
6. DiPiro JT, Patrias JM, Townsend RJ, et al. Oral neomycin sulfate and erythromycin base before colon surgery: A comparison of serum and tissue concentrations. Pharmacotherapy 1985;5:91-4. [ Links ]
7. Eltawil KM, Laryea M, Peltekian K, et al. Rifaximin vs. conventional oral therapy for hepatic encephalopathy: A meta-analysis. World J Gastroenterol 2012;18:767-77. DOI: 10.3748/wjg.v18.i8.767. [ Links ]
8. Alimonti A, Satta F, Pavese I, et al. Prevention of irinotecan plus 5-fluorouracil/leucovorin-induced diarrhoea by oral administration of neomycin plus bacitracin in first-line treatment of advanced colorectal cancer. Ann Oncol 2003;14:805-6. DOI: 10.1093/annonc/mdg193. [ Links ]
9. Spiller R, Lam C. The shifting interface between IBS and IBD. Curr Opin Pharmacol 2011;11:586-92. DOI: 10.1016/j.coph.2011.09.009. [ Links ]
10. Grasa L, Abecia L, Forcen R, et al. Antibiotic-induced depletion of murine microbiota induces mild inflammation and changes in toll-like receptor patterns and intestinal motility. Microb Ecol 2015;70:835-48. DOI: 10.1007/s00248-015-0613-8. [ Links ]
11. Patel RM, Myers LS, Kurundkar AR, et al. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol 2012;180:626-35. DOI: 10.1016/j.ajpath.2011.10.025. [ Links ]
12. Ren T, Grants I, Alhaj M, et al. Impact of disrupting adenosine A(3) receptors (A(3)(-)/(-) AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis 2011;17:1698-713. DOI: 10.1002/ibd.21553. [ Links ]
13. Fujita T, Kawahara I, Quan Y, et al. Permeability characteristics of tetragastrins across intestinal membranes using the Caco-2 monolayer system: Comparison between acylation and application of protease inhibitors. Pharm Res 1998;15:1387-92. DOI: 10.1023/A:1011997404306. [ Links ]
14. Rull S, Ponz F. Effects of neomicine on the transport of sugars by rat and hamster small intestine (author's transl). Rev Esp Fisiol 1974;30:183-90. [ Links ]
15. De Lisle RC, Mueller R, Boyd M. Impaired mucosal barrier function in the small intestine of the cystic fibrosis mouse. J Pediatr Gastroenterol Nutr 2011;53:371-9. DOI: 10.1097/MPG.0b013e318219c397. [ Links ]
16. Xu D, Gao J, Gillilland M, 3rd, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014;146:484-96 e4. DOI: 10.1053/j.gastro.2013.10.026. [ Links ]
17. Holmes JL, Van Itallie CM, Rasmussen JE, et al. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 2006;6:581-8. DOI: 10.1016/j.modgep.2005.12.001. [ Links ]
18. Laukoetter MG, Nava P, Lee WY, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med 2007;204:3067-76. DOI: 10.1084/jem.20071416. [ Links ]
19. Al-Sadi R, Khatib K, Guo S, et al. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 2011;300:G1054-64. DOI: 10.1152/ajpgi.00055.2011. [ Links ]
20. Liu H, Wang P, Cao M, et al. Protective role of oligomycin against intestinal epithelial barrier dysfunction caused by IFN-gamma and TNF-alpha. Cell Physiol Biochem 2012;29:799-808. DOI: 10.1159/000188076. [ Links ]
21. Garrido-Mesa N, Utrilla P, Comalada M, et al. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem Pharmacol 2011;82:1891-900. DOI: 10.1016/j.bcp.2011.09.004. [ Links ]
22. Hwang I, An BS, Yang H, et al. Tissue-specific expression of occludin, zone occludens-1, and junction adhesion molecule A in the duodenum, ileum, colon, kidney, liver, lung, brain, and skeletal muscle of C57BL mice. J Physiol Pharmacol 2013;64:11-8. [ Links ]
![]() Correspondence:
Correspondence:
Laura Grasa.
Departamento de Farmacología y Fisiología.
Facultad de Veterinaria.
Universidad de Zaragoza.
Miguel Servet, 177.
50013 Zaragoza, Spain
e-mail: lgralo@unizar.es
Received: 27-05-2015
Accepted: 25-07-2015











 text in
text in