My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 n.4 Madrid Apr. 2016
Proton-pump inhibitors adverse effects: a review of the evidence and position statement by the Sociedad Española de Patología Digestiva
Efectos adversos de los inhibidores de la bomba de protones: revisión de evidencias y posicionamiento de la Sociedad Española de Patología Digestiva
Cristóbal de-la-Coba1,2, Federico Argüelles-Arias3, Carlos Martín-de-Argila4, Javier Júdez5, Antonio Linares6, Aida Ortega-Alonso7, Enrique Rodríguez4, Manuel Rodríguez-Téllez8, Isabel Vera9, Lara Aguilera4, Ángel Álvarez10, Raúl J. Andrade7, Fidencio Bao11, Manuel Castro12 and Froilán Giganto13, on behalf of SEPD
1 Coordinador Comité de Excelencia Clínica. SEPD. Spain.
2 Digestive Diseases Unit. Hospital de Cabueñes. Gijón. Spain.
3 UGC Digestive Diseases. Hospital Universitario Virgen Macarena. Sevilla, Spain.
4 Department of Gastroenterology and Hepatology. Hospital Universitario Ramón y Cajal. Madrid, Spain.
5 Department of Knowledgement Management. SEPD. Spain.
6 Department of Digestive Diseases. Sanatorio Nuestra Señora de Covadonga. Gijón, Asturias. Spain.
7 UGC Digestive Diseases. Instituto de Investigación Biomédica de Málaga (IBIMA). Hospital Universitario Virgen de la Victoria. Universidad de Málaga. Málaga, Spain. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd). Madrid, Spain.
8 UGC Digestive Diseases. Hospital Universitario Virgen Macarena. Universidad de Sevilla. Sevilla, Spain.
9 Department of Digestive Diseases. Hospital Universitario Puerta de Hierro. Madrid, Spain.
10 Department of Digestive Diseases. Hospital Clínico San Carlos. Universidad Complutense de Madrid. Madrid, Spain.
11 Digestive Unit. Hospital San Eloy. Barakaldo, Vizcaya, Spain.
12 Department of Digestive Diseases. Hospital Universitario de Valme. Sevilla, Spain.
13 Department of Digestive Diseases. Hospital Central de Asturias. Oviedo, Asturias, Spain
ABSTRACT
Introduction: In the last few years a significant number of papers have related the use of proton-pump inhibitors (PPIs) to potential serious adverse effects that have resulted in social unrest.
Objective: The goal of this paper was to provide a literature review for the development of an institutional position statement by Sociedad Española de Patología Digestiva (SEPD) regarding the safety of long-term PPI use.
Material and methods: A comprehensive review of the literature was performed to draw conclusions based on a critical assessment of the following: a) current PPI indications; b) vitamin B12 deficiency and neurological disorders; c) magnesium deficiency; d) bone fractures; e) enteric infection and pneumonia; f) interactions with thienopyridine derivatives; e) complications in cirrhotic patients.
Results: Current PPI indications have remained unchanged for years now, and are well established. A general screening of vitamin B12 levels is not recommended for all patients on a PPI; however, it does seem necessary that magnesium levels be measured at therapy onset, and then monitored in subjects on other drugs that may induce hypomagnesemia. A higher risk for bone fractures is present, even though causality cannot be concluded for this association. The association between PPIs and infection with Clostridium difficile is mild to moderate, and the risk for pneumonia is low. In patients with cardiovascular risk receiving thienopyridines derivatives it is prudent to adequately consider gastrointestinal and cardiovascular risks, given the absence of definitive evidence regardin potential drug-drug interactions; if gastrointestinal risk is found to be moderate or high, effective prevention should be in place with a PPI. PPIs should be cautiously indicated in patients with decompensated cirrhosis.
Conclusions: PPIs are safe drugs whose benefits outweigh their potential side effects both short-term and long-term, provided their indication, dosage, and duration are appropriate.
Key words: Proton-pump inhibitors. Adverse effects. Guidelines as topic. Position statement. SEPD.
RESUMEN
Introducción: en los últimos años, numerosos artículos relacionan el uso de los inhibidores de la bomba de protones (IBP) con posibles efectos adversos serios que han creado cierta alarma social.
Objetivo: el objetivo de este trabajo es revisar la literatura de cara a elaborar un documento institucional de posicionamiento de la Sociedad Española de Patología Digestiva (SEPD) sobre la seguridad de los IBP a largo plazo.
Material y métodos: se ha realizado una revisión exhaustiva de la literatura orientada a la presentación de conclusiones tras una valoración crítica sobre los siguientes temas: a) indicaciones actuales de los IBP; b) déficit de vitamina B12 y alteraciones neurológicas; c) déficit de magnesio; d) fracturas óseas; e) infecciones entéricas y neumonías; f) interacción con los derivados de las tienopiridinas; y e) complicaciones en pacientes cirróticos.
Resultados: las indicaciones actuales de los IBP no han variado en los últimos años y están bien establecidas. No se recomienda la realización de un cribado generalizado de los niveles de vitamina B12 en todos los pacientes tratados de forma crónica con estos medicamentos; sin embargo, sí parece necesario controlar los niveles de magnesio al inicio del tratamiento y monitorizarlos en pacientes con toma de otros fármacos que puedan inducir hipomagnesemia. Existe mayor riesgo de fracturas óseas, aunque no se puede concluir que esta asociación sea causal. La asociación IBP e infección por Clostridium difficile es débil o moderada y el riesgo de neumonía es bajo. En pacientes con riesgo cardiovascular y tratados con derivados de las tienopiridinas -dada la ausencia de evidencias definitivas en relación a posibles interacciones medicamentosas- parece que lo prudente sea sopesar adecuadamente los riesgos gastrointestinales y los riesgos cardiovasculares de cada paciente; cuando el riesgo gastrointestinal sea moderado/alto, debemos ejercer una acción terapéutica de prevención efectiva utilizando un IBP. En cirróticos descompensados deben ser indicados con cautela.
Conclusiones: los IBP son fármacos seguros y los beneficios de su empleo, tanto a corto como a largo plazo superan los posibles efectos secundarios, siempre que la indicación, dosis y duración sean las adecuadas.
Palabras clave: Inhibidores de la bomba de protones. Efectos adversos. Guía clínica. Documento de posicionamiento. SEPD.
Introduction
Proton-pump inhibitors (PPI) are drugs widely used in Spain. They irreversibly inhibit the enzyme H/K-ATPase in the parietal cells of the gastric mucosa, thus reducing acid secretion. While PPIs have a short half-life (1-2 hours), this irreversible inhibition provides a longer effect, as new proton pumps need be synthesized before acid secretion is resumed (1).
PPIs are among the most commonly prescribed drugs and their turnover ranks high in the National Health System. PPI use in Spain has increased significantly in the past few years, going from 21.8 daily doses per 1,000 population in 2,000 to 96.57 daily doses per 1000 population in 2008 (2), with omeprazole becoming the most widely used drug in Spain in 2010 in terms of package count (3). Between 2000 and 2008 PPI prescriptions increased by 200% (3), and from 2004 to 2010 PPI use increased by 227%; however, the cost for public funds only increased by 21.3%, which represents a total of approximately €626 million (4). This implies a reduction in cost per package, likely because of generics, but public spending remains unchanged or even increases given a remarkable increase in prescriptions (4). Furthermore, when compared to other European countries, 85 in every 1000 people use a PPI daily in Spain, versus only 30 per 1000 in Norway and 27 per 1000 in Italy (2).
On the other hand, a number of studies reported during the last few years have associated PPI use with various adverse events, which has risen concern among both prescribers and patients. A significant need for a review on the use and side effects of PPIs in our setting was identified by Sociedad Española de Patología Digestiva (SEPD). It is not uncommon for patients receiving a PPI prescription to seek medical advice on the potential adverse effects of their prescribed drug and its long-term use.
Therefore, the goal of this paper is to identify, assess, and review the major evidence regarding the adverse effects associated with long-term PPI use, and to write a position document of SEPD including clear conclusions on the safety of this class of drugs.
Material and Methods
In order to perform the present review a task force made up of gastroenterologists selected, based on a literature search, the most relevant topics related to PPIs and their adverse effects. These include: a) Indications and rationale for PPI use; b) vitamin B12 deficiency and neurological changes; c) magnesium deficiency; d) bone fractures; e) enteric infection and pneumonia; f) use of PPIs together with thienopyridine derivatives and cardiovascular risk; and e) complications in cirrhotic patients. Each question was answered according to the best evidence currently available following a comprehensive search of the PubMed, EMBASE, and Cochrane Library databases. After an initial draft and an in-person meeting at the 2015 SEPD Conference in Seville, a revised text was submitted to reach the highest agreement possible. In this process also a knowledge management expert played a role. Finally, the document was externally reviewed by the SEPD Executive Board.
Indications and rationale for PPI use
Five PPI types are currently marketed with the following names, standard doses (milligrams), and administration routes (PO: oral, IV: intravenous) (Tables I and II): omeprazole 10 and 20 mg (PO), 40 mg (PO and IV); lansoprazole 15 and 30 mg (PO); pantoprazole 20 and 40 mg (PO), 40 mg (IV); rabeprazole 10 and 20 mg PO); esomeprazole 10, 20 and 40 mg (PO), 40 mg (IV), with omeprazole being first on the market, cheaper, and most widely used (3).
The reason why PPIs are prescribed in Spain at 70% above the european average is most likely related to inappropriate prescription, and the most widely mistaken indication possibly is the prophylaxis of gastroduodenal injury in patients with low (even nil) gastrolesive risk, as has been shown in other countries (5-7). Furthemore omeprazole represents 75% of PPI prescriptions, with the remaining 25% amounting to 75% of expense, which reflects the significance of using a specific PPI in addition to appropriate indication, route, dosage, and duration (2).
It is thought that 54% to 69% of PPI prescriptions are incorrect (8-16), with hospital admision being, for example, a risk factor. A study in a Spanish tertiary referral hospital (15) found that 28.7% of patients were already using a PPI before admission, 82.6% received a PPI during their hospital stay, and 54.8% had a PPI recommended on discharge. Prescription was deemed inappropriate in 74.5%, 61.3%, and 80.2% of patients, respectively. In a study recently reported by the Revista Española de Enfermedades Digestivas (14), PPI indication was considered inappropriate for 63.6% of inpatients, mostly because of an unnecessary inclusion of these drugs in surgical protocols, in diagnostic or therapeutic procedures, or for the management of disease. A review of discharge reports usually reveals no information warranting a recommendation for ongoing therapy with a PPI in 54.5% of individuals; indication is uncertain for 12.7%, and evidence-based for only 32.7% of patients (12). In a later study to assess prescription 6 months before and after hospital discharge data were replicated, and on-discharge PPI indication was found to be inappropriate in 52% of cases, appropriate in only 35% of patients, and uncertain for 13%; of these, 58%, 67%, and 73%, respectively, maintained their PPI prescription after discharge. The fact that two thirds of inappropriate prescriptions originated within a hospital is to be highlighted (16). Inappropriate PPI prescription is a common issue involving all levels of care.
Accordingly, clear indications to be followed as much as possible are a key factor. Current recommendations are listed in Table 3 (17-19).
A PPI prescription in association with a nonsteroidal anti-inflammatory drug (NSAID) is recommended for patients with a history of peptic ulcer disease or gastrointestinal (GI) bleeding, 60 years of age or older, with severe comorbidities, on a high-dose NSAID, and using a concomitant second NSAID (including low-dose acetylsalicylic acid or ASA) or anticoagulant, antiaggregant or glucocorticoid (20). A potentially controversial indication is the prophylaxis of stress ulcers. It is currently recommended for patients in an intensive care unit (ICU) who also exhibit an additional risk factor such as history of peptic ulcer, renal failure, coagulopathy, shock or serious sepsis, need for mechanical ventilation, brain trauma, burns, or neurosurgical procedures (21). For peptic ulcer-related GI bleeding PPIs have been shown to reduce bleeding when compared to placebo (22). As regards their schedule, major clinical guidelines recommend continuous infusion at 8 mg/hour; however, two recently reported studies have shown that the effectiveness of PO and IV bolus administration is comparable to that of continuous infusion (23,24). For uninvestigated and functional dyspepsia PPIs represent a treatment alternative to Helicobacter pylori eradication and upper digestive endoscopy (25). Finally, PPIs are used to differentiate eosinophilic esophagitis from gastro-esophageal reflux disease (GERD) and PPI-responsive esophageal eosinophilia (26,27), a condition for which they are now being considered a first-line therapy (27,28). In exocrine pancreatic insufficiency not responsive to isolated pancreatic enzymes, a PPI provides improved fat digestion (29). According to the above, currently a PPI may only be recommended for the aforementioned conditions.
May PPIs induce vitamin B12 deficiency and neurologic disturbances?
Vitamin B12 or cobalamin plays a key role in the synthesis of myelin and several myelopoiesis stages. It is found mainly in animal source food bound to various protein compounds. Vitamin B12 separation from food in the stomach is a key step allowing its binding to intrinsic factor and subsequent absorption in the terminal ileum (30). Pepsin acts as a catalyzing enzyme for this process, and only becomes active when gastric pH is lower than 4. PPIs have been said to potentially induce a deficient absorption of this vitamin by reducing gastric juice acidity.
Available evidence supporting this association mainly derives from in vitro data, experimental research on small samples, observational studies, and a 2015 systematic review and metaanalysis including 5 observational studies (31-40).
The paper by Lam, et al. stands out in terms of sample size and impact on the scientific community (32). This case-control study included 25,956 patients over 18 years of age who were diagnosed with vitamin B12 deficiency, and 184,199 paired healthy controls. Potential confounders taken into account included diagnosis with diabetes mellitus, thyroid disease, Helicobacter pylori infection, atrophic gastritis, use of drugs potentially associated with vitamin B12 deficiency such as metformin, etc. In both groups, the use of PPIs (OR: 1.65; 95% CI: 1.58-1.73) or H2 antagonist (anti-H2) drugs (OR: 1.25; 95% CI: 1.17-1.34) for 2 or more years was associated with a higher risk for vitamin B12 deficiency. Other findings included a dose-dependent effect and a decrease in association magnitude after drug discontinuation (p = 0.007). However, this paper did not assess diet as potential confounder, case definition was inconsistent, limitations entailes by serum cobalamin levels as a marker of B12 deficiency were not counted in, over-the-counter use of PPIs (common in the USA) in both groups was not discussed, and PPI dosage was measured in tablets rather than milligrams even though different PPI tablets not always contain equivalent doses.
Furthermore, a relevant number of studies has found no association between PPI use and vitamin B12 deficiency (41-48). Recently, the results of a detailed analysis of adverse effects in the LOTUS (Long-Term Usage of Esomeprazole vs. Surgery for Treatment of Chronic GERD) (n = 514) and SOPRAN (Safety of Omeprazole in Peptic Reflux Esophagitis: A Nordic Open Study) (n = 298) clinical trials were reported. With 5 and 12 years, respectively, of PPI use, no significant differences in serum vitamin B12 levels were found between treatment and control groups (48). The first case reported of a clinical abnormality directly related to vitamin B12 deficiency secondary to PPI use (omeprazole 40-60 mg for 4 years for the management of complicated GERD in association with peptic esophagitis and megaloblastic anemia) (49) has not been consistently replicated. Hence, despite potential risk for anemia and neurologic damage as a result of vitamin B12deficiency from chronic PPI use, such potential association has not been shown to be clinically relevant. PPIs might also induce neurologic damage through a mechanism unrelated to vitamin B12 deficiency. In this respect, a case-control study associating chronic PPI use with risk for any dementia (hazard ratio: 1.38, 95% CI: 1.04-1.83) and risk for Alzheimer's disease (HR: 1.44, 95% CI: 1.01-2.06) was reported at the end of 2014 (50). Isolated cases of peripheral neuropathy reversible on therapy discontinuation may also be found in the literature (51,52).
From all the above, the limited amount and quality of the available evidence does not allow to conclude that chronic PPI use be a risk factor for neurological damage as derived from vitamin B12 deficiency.
Finally, the measurement of serum vitamin B12 levels to assess body storage has multiple limitations. Sensitivity for values < 200 pg/mL is 65-95%, with an estimated specificity of 50%. Overall, false negative and false positive rates are deemed to be around 50% (30). Homocysteine and methylmalonic acid levels increase during vitamin B12 deficiency, and have a higher diagnostic value. Anytime vitamin B12 deficiency (< 300-350 pg/mL) is suspected on clinical or laboratory grounds, testing should be expanded to include both measurements (30).
In conclusion, in the light of current evidence a generalized screening of vitamin B12 levels cannot be advised for all patients on chronic PPI therapy. For advanced age individuals, particularly those with risk factors for this vitamin deficiency, including Crohn's disease, history of gastric/intestinal surgery, pernicious anemia, stringent vegetarianism, and malnutrition, an assessment of cobalamin stores seems appropriate within 2-3 years after therapy onset, with follow-up yearly or every two years, and vitamin replacement therapy as needed. In any case, prospective studies specifically designed to assess the real extent of a potential association between chronic PPI use and vitamin B12 deficiency are needed.
And magnesium deficiency?
Blood magnesium levels are dependent upon balance between intestinal absorption and renal excretion. Hypomagnesemia usually develops from decreased ingestion or absorption, increased loss (whether urinary or gastrointestinal), or impaired transportation. Hypomagnesemia has been recently associated with long-term PPI use (53-56). This is an adverse effect of unknown prevalence that raised significant controversy among prescribers. While the biological mechanism of PPI-related hypomagnesemia remains partly unknown, the increased pH induced by these drugs would seemingly affect transient receptor potential melastatin type 6 and 7 channels, thus reducing magnesium active transport and absorption (57).
Although PPIs were initially marketed in 1989, the first case report on PPI use and hypomagnesemia dates back to 2006 (58). Several observational studies followed, which assessed the potential association between PPI use and hypomagnesemia with conflicting results (54,55) or very low prevalence findings (56). Some were criticized for lack of consideration of patient diet; furthermore, since magnesium is an intracellular ion, serum concentrations do not reflect total magnesium, which renders measurement a challenging procedure. Despite low study consistency, in 2011 the "Agencia Española de Medicamentos y Productos Sanitarios" (AEMPS), the U.S. Food and Drug Administration (FDA), and the Australian Medicines Safety Update of Therapeutic Goods Administration published an informative note advising that such diagnostic possibility be assessed should suggestive symptoms develop during prolonged PPI use. The note also recommended magnesium testing at baseline and then regularly under certain conditions (59-61). Later, a systematic review and meta-analysis in 2014 (62) revealed an association between PPI use and hypomagnesemia; however, high heterogeneity between studies prevented a definitive conclusion. In the cross-sectional study by Luk (63), which included 693 patients on PPIs with hypomagnesemia, the risk for hypomagnesemia was seen to vary among PPIs, being highest for pantoprazole and lowest for esomeprazole. Also the recent systematic review and meta-analysis of observational studies by Cheungpasitporn (64) found a statistically significant risk for the association of PPIs and hypomagnesemia. Current theories regarding the mechanism of PPI-related hypomagnesemia do not explain risk differences between PPIs. To date, no study has been carried out to compare hypomagnesemia risk differences between different PPIs.
In the studies reporting on impaired magnesium absorption with PPI use, patients had received a PPI for at least one year, which suggests that short-term PPI use does not reduce magnesium levels. Hypomagnesemia may be asymptomatic or result in vomiting, diarrhea, and even tetany, confusion and seizures. It is furthermore associated with prolonged QT in the ECG and electrolyte imbalance, including hypocalcemia and hypokalemia. While the frequency of this adverse event, which may be overlooked, remains unknown, both the AEMPS and FDA recommend magnesemia monitoring at treatment onset, and that follow-up be considered, particularly for prolonged PPI use in patients on concomitant PPIs and other hypomagnesemia-inducing drugs, including loop or thiazide diuretics, and drugs potentially affected by serum magnesium levels, such as digoxin (57,59,60). Other patients, including individuals with advanced age, diabetes mellitus, renal failure, and cardiovascular conditions, may also benefit from regular magnesium testing. The management of patients on a PPI with hypomagnesemia must be individualized, and the drug should be discontinued when the indication is inappropriate. Should its administration be advisable, it is recommended that minimum doses and magnesium supplementation be administered, even though this regimen has never been prospectively assessed.
Do PPIs increase bone fracture risk?
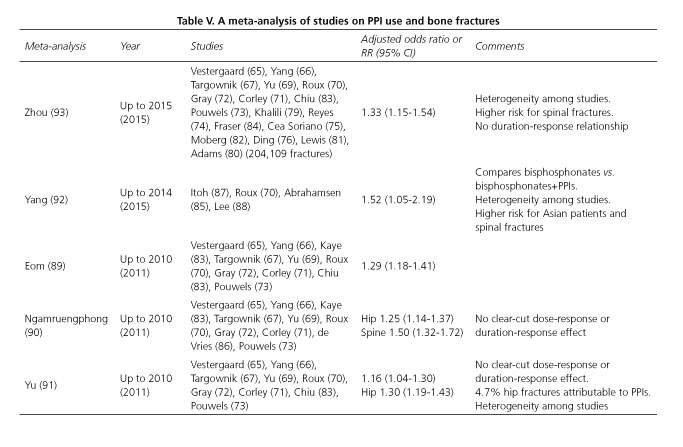
Multiple observational studies (65-88) and meta-analyses (89-93) have assessed PPI use, either alone or in combination with bisphosphonates, in relation to bone fracture risk. In these studies PPI use is associated with a higher risk for fragility bone fractures as a result of osteoporosis, particularly involving the spine and hip (Table IV -studies on PPIs and bone fractures-, and Table V -meta-analyses of studies on PPIs and bone fractures-). The strength of this association is low, somewhat greater when adherence is high (76) or for higher daily doses of PPI (71,73,75,85). However, although likely, no dose-response or duration-response association has been well established (72,75,80,86).
This weakness suggests a potential role of confounding factors. Kaye (68) performed a study where all patients with medical conditions associated with hip fracture risk were excluded, and found no increased risk for PPI use: RR 0.99 (95% CI: 0.7-1.1). Age, female sex, body mass index, alcohol and tobacco use, history of prior falls or fractures, neurological and hematological disease, comorbidities, and use of drugs such as antidepressants, anxiolytics, antipsychotics, antiepileptics, diuretics, and antidiabetics represent confounding factors that modulate PPI-related bone fracture risk (68,74-76,81,82,89,94,95).
Most studies have been performed in Nordic or Anglosaxon countries, where a high prevalence of fragility fractures is present -as is well known, prevalence in Spain is lower. In a retrospective case-control study in Catalonia (74) PPI use was associated with fracture risk with an OR of 1.44 (95% CI: 1.09-1.89; p = 0.009), which disappears after adjusting for confounding factors. In an also Catalan population cohort (96) including patients who received bisphosphonates, with 21,385 subjects of whom 2,026 had at least one fracture, PPIs were associated with fracture risk -OR 1.41 (95% CI: 1.22-1.65; p < 0,001).
Virtually no clinical trials are available to complete the information provided by observational studies. Itoh (87) compared risedronate versus risedronate pluas rabeprazole (10 mg), and found no differences in fracture numbers between groups, but the study was not designed for that purpose. The aforementioned SOPRAN and LOTUS studies also found no differences in fracture numbers (48).
At any rate, no mechanism has been found through which PPIs would presumably induce fractures. Its use has not been shown to reduce calcium absorption (48,97,98) or induce significant changes in bone mineral density (69,70,72,87,99-101), and some study suggests that proton pump inhibition in osteoclasts by PPIs may impair bone remodeling (102).
Although osteoporosis-related bone fragility fractures entail a significant socio-economic impact and high morbidity and mortality rates, only 1% to 5% may be attributed to PPI use (91,103). Therefore, clinical relevance seems low. In this respect, the FDA points out that evidence is inadequate to recommend calcium supplementation or regular bone density scans (60).
In conclusion, PPI use is associated with higher risk for bone fractures, but such association cannot be said to be causal. Available evidence does not allow to recommend PPI discontinuation in order to prevent bone fractures, but inappropriate prescription must be precluded, and minimum effective doses are to be sought (60,104).
Do PPIs favor enteric infection and pneumonia?
The available scientific evidence, based on case-control and cohort studies, suggests that PPI is associated with a mildly increased risk of enteric infection, particularly with Clostridium difficile (CD), and community-acquired pneumonia (CAP). Gastric acid represents a physiological antimicrobial barrier. Infection risk during PPI use results from gastric secretion inhibition, that is, the beneficial effect of these drugs.
Leonard (105) used a meta-analysis to assess the association between enteric infection and gastric antisecretory drugs. The use of these drugs was associated with increased risk for infection with Salmonella, Campylobacter, and additional organisms other than CD (OR 2.55; 95% CI: 1.53-4.26), the association being higher for PPIs (OR 3.33; 95% CI: 1.84-6.02) than for anti-H2 agents (OR 2.03; 95% CI: 1.05-3.92). Two recently reported studies associated PPI use with Salmonella (106, 107). García-Rodríguez (108) carried out a case-control study in Spain, which also identified a greater risk for infection with these organisms during PPI use (RR 2.9; 95% CI: 2.5-3.5) but not during therapy with anti-H2 agents (RR 1.1; 95% CI: 0.9-1.4). The association between PPI use and CD infection has been most studied. Three meta-analyses reported in 2012 (109-111) identified a greater risk for CD infection (OR 1.74; 95% CI: 1.47-2.85; OR 2.31; 95% CI: 1.72-3.10 for cohort studies, and OR 1.48; 95% CI: 1.25-1.75 for case-control studies; and OR 2.15; 95% CI: 1.81-2.55, respectively).
In the meta-analysis by Kwok (109) the risk associated with PPI use (OR 1.74; 95% CI: 1.47-2.85) increased with concurrent antibiotic use (OR 1.96; 95% CI: 1.03-3.70). The causal association between PPI use and CD infection may be considered mild to moderate. Most studies provide no information on the influence of variables such as treatment duration, comorbidity, hospitalization, advanced age, etc., hence recommendations are challenging regarding preventive measures in clinical practice. However, we should be particularly cautious in prescribing PPIs for patients with risk factors for CD infection -a recently reported retrospective study (112) concluded that PPIs should be avoided in patients with recurrent CD infection. In this regard, the FDA recommends that the diagnosis with CD-related diarrhea be considered for patients on PPIs with persistent diarrhea. Furthermore, given the risk for infection with CD, they recommend that PPIs be prescribed at the lowest effective dose for the shortest duration possible (113).
As regards the association of pneumonia and PPI use, case-control studies reported in 2004-08 (114-116) and subsequent meta-analyses (117-120) suggest that PPI use may be associated with a mildly increased risk of CAP. In the review by Lambert (119) an OR of 1.49 (95% CI: 1.16-1.92) was found. A pathogenic relationship has not been well established but might involve gastric bacterial colonization, changes in oropharyngeal bacterial flora, and pulmonary microaspiration. The fact should be highlighted that increased risk is related to treatment duration, being higher within 1 month after therapy onset (OR 2.10; 95% CI: 1.39-3.16) regardless of dosage and patient age. It is also associated with an increased risk of hospitalization for CAP (OR 1.61; 95% CI: 1.12-2.31).
In summary, the increased CAP risk reported during therapy with PPIs is low, and only relevant for short-term regimens, with no convincing explanation. The absence of higher quality studies, as occurs with enteric infection, hinders the assessment of causal associations and a most likely influence of confounding factors (121,122). The information available does not allow recommendations on CAP prevention in clinical practice, except using PPIs for stringent indications alone.
Is PPI use concurrently with thienopyridine derivatives safe, or do they increasecardiovascular risk?
Clopidogrel and ticlopidine are thienopyridine derivatives with platelet antiaggregation activity entailing a higher risk for gastrointestinal bleeding, particularly in patients with a history of digestive bleeding and peptic ulcer. Clopidogrel is now more commonly used, and undergoes conversion to its active metabolite in the liver primarily by cytochrome P450 isoenzymes CYP2C19 and CYP3A4. These isoenzymes also play a role in PPI metabolism, this being why PPIs inhibit clopidogrel activation (123,124). Prasugrel is a novel thienopyridine that only requires a metabolic step via the hepatic CYP isoenzymes to become an active compound. Therefore, platelet inhibition is faster, more pronounced with lower doses, and response is less variable. Also, prasugrel response is less affected by concomitant CYP inhibitors (125).
The first study who drew attention on the interaction of omeprazole (20 mg daily) and clopidogrel (75 mg daily) was reported by Gilard (126). The percentage of platelet function inhibition after 7 days on both drugs was much lower in the omeprazole group (39.8 ± 15.4%) versus the placebo group (51.4 ± 16.4%, p < 0.0001). This study did not analyze the potential clinical consequences of this effect. While many observational studies are available, few randomized controlled studies (127-129) and meta-analyses (130-133) allow to assess the clinical effect of concomitant PPI use on clopidogrel efficacy.
The COGENT study (127) compared clopidogrel versus clopidogrel + omeprazole (20 mg). It included 3,873 patients either with acute coronary syndrome or undergoing percutaneous revascularization. No differences in cardiovascular events were reported between both groups, but upper GI bleeding rates were lower among those treated with omeprazole. The TRITON-TIMI study (128) included 13,608 patients, and showed no association between PPI use and cardiovascular risk. Another study, PLATO (129), included 18,624 patients receiving clopidogrel or ticagrelor. Although a mildly increased cardiovascular risk was reported for those on PPIs, such risk was also seen among those on other drugs, including anti-H2 agents; hence the authors concluded that PPI use may be considered a cardiovascular risk marker rather than its cause.
As regards the meta-analyses reported thus far (130-133), most studies included are observational in nature, whether retrospective or prospective. Their findings are conflicting in that some of them show a marked increase in serious cardiovascular adverse events, whereas others only find a mild or even absent increase or risk of readmission.
The most recent one, by Kwok (133), included 23 studies in 222,311 patients. While the pooled cardiovascular risk was significantly increased among subjects on PPIs, no differences were shown between PPIs, including pantoprazole, reflecting their different pharmacokinetics and their related platelet function assessments; the authors concluded that confounders and biases likely play a role in many of the pooled studies.
Several international agencies and expert committees have taken a stance on this subject. The EMA (European Medicines Agency) published an alert in May 2009 (amended in March 2010) suggesting that clopidogrel may be less effective in patients on PPIs, which would increase the risk of cardiovascular adverse effects (134,135). The FDA pronounced themselves similarly in 2010 (136), and the AEMPS published a warning advising against the use of omeprazole and esomeprazole -but not other PPIs- combined with clopidogrel (137). The Canadian Cardiology Society (2011) and the European Cardiology Society (2013) recommend that PPIs with less inhibition of CYP2C19 be used in patients on clopidogrel with high risk for upper GI bleeding (138,139).
Complexity has grown following a recently reported observational study (140) where a novel data mining technique was use to recover information from electronic clinical records. The primary analysis included 70,477 patients with gastroesophageal reflux disease, and the authors concluded that PPI use is associated with myocardial infarction -OR 1.16 (95% CI: 1.09-1.24). The increase seen in absolute risk is moderate- only one in 4,000 patients on a PPI may experience myocardial infarction. This effect is independent of clopidogrel use, hence the increase in cardiovascular events seen in patients receiving clopidogrel plus a PPI may also be accounted for by a PPI class effect. The weakness of this association, the differences between PPIs, the lack of control for confounders and independent cardiovascular risk factors, and the information collection approach advise caution in interpreting the study's findings.
The SOPRAN study (141), a randomized, multicenter, prospective trial in patients followed up for 12 years, reported 8 myocardial infarction events in the omeprazole group versus 2 in the surgical procedure group. However, this is attributed to older mean age and more prior MIs in the omeprazole group, as well as more losses to follow-up in the surgical procedure group.
During 2015, at least 5 additional sudies have been reported (142-146) that attempt to correlate PPI plus clopidogrel use with higher CV risk, with no conclusive findings. While observational studies suggest a PPI-clopidogrel interaction, such interaction remains clinically unidentified in randomized clinical trials (147-149).
Therefore, based on the currently available data, patients with acute coronary syndrome or undergoing coronary artery revascularization on thienopyridines should only be prescribed a PPI when the indication is clear, particularly if a history of peptic ulcer or upper GI bleeding is present. Anyway, caution must be exerted, and further prospective, randomized studies are needed to shed light on this potential interaction.
May PPIs increase complication risk in patients with liver cirrhosis?
PPIs entered clinical practice in the 1980s, and have been widely used in patients with liver cirrhosis ever since for multiple indications (peptic ulcer, gastroesophageal reflux, portal hypertensive gastropathy, post-sclerotherapy ulcer prevention, digestive bleeding, and Barrett's esophagus, among others). Considered from the outset as virtually harmless drugs, various studies reported in recent years warn against infections related to PPI use, primarily nosocomial pneumonia (150), infection with Clostridium difficile (151), and spontaneous bacterial peritonitis (SBP) (152,153).
Numerous studies have suggested that PPI use in patients with cirrhosis increases SBP risk, whereas others have found the opposite (152-166) (Table VI). Two pioneering meta-analyses (155,158) supporting the former hypothesis have been criticized for low methodological quality, namely the inclusion of a reduced number of studies and lack of any bias identification approach. A recent meta-analysis by Xu (153) concluded that these drugs, when used in patients with cirrhosis and ascites, significantly increase SBP risk, with OR 2.17 (95% CI: 1.46-3.23), albeit with high heterogeneity (I2 = 85.6%). In contrast, a simultaneously reported multicenter study (165) carried out in Argentina on 521 patients refutes this hypothesis, since no differences in PPI use were found for patients developing a SBP event or otherwise. However, the latter study, which was reported as prospective, was based on a survey on PPI use in the prior 3 months that was administered to patients on hospital admission.
Regarding dosage, an increased risk has been described for standard versus halved doses (162) (OR: 2.184; 95% CI: 0.935-5.103; p = 0.07). Comparisons are challenging for treatment duration since no consistent definitions are found among studies. With this limitation, both increased risk for SBP with longer treatment (167) and no influence of this variable have been reported (157).
Regarding bacterial infection risk, the meta-analysis found it increased, with OR 1.98 (95% CI: 1.36-2.87; p < 0.05) (153). The study by Merli (168) suggests that chronic therapy with PPIs increases the prevalence of infection, particularly in patients with severe liver disease, hospitalized within the past 6 months, or having experienced infection during the previous year. Experimental studies in animal models (rats) showed that PPIs induce bacterial overgrowth in the gut. However, the factor predominantly determining infection risk in cirrhotic patients is function stage, with a 3-fold higher probability for Child-Pugh grades B and C, as compared to grade A; increased intestinal permeability by these drugs may be a contributing factor (169), a hypothesis supported by other studies (163).
A recent single-center, prospective study (170) related PPI use with increased mortality risk in a cohort of 272 cirrhotic patients (OR 2.363; 95% CI: 1.264-4.296; p = 0.007 for the multivariate analysis), although patients on PPIs had greater impairment of liver function (Child-Pugh, MELD) when compared to the other group (84% vs. 59.3% were Child B-C). In the univariate study, PPIs were related to colonization with multiresistant bacteria, a trend that persisted in the multivariate analysis albeit without reaching statistical significance.
Future prospective, well designed studies are needed to clarify the association of different PPIs with infection risk in cirrhotic patients, as well as the potential effect of dose and duration on said risk.
In summary, PPI use in patients with liver cirrhosis, particularly those with cirrhotic decompensation, may have a deleterious effect, and therefore should be cautiously indicated on an individual basis in this subgroup. Acid secretion is reduced in these patients, hence evidence is inadequate to indicate PPIs as prophylaxis for peptic complications in patients portal hypertensive gastropathy or esophageal varices.
Discussion
Overall, we may assert that PPIs represent a safe drug class with mostly mild adverse effects including headache, constipation, diarrhea, dyspepsia, skin rash, and rarely symptomatic hypomagnesemia. No cases of cancer or carcinoid tumors have been reported in association with long-term PPI use. Drug-drug interactions may occur through various mechanisms, including impaired absorption from gastric pH changes, or through competition for cytochrome P450. Significant interactions include atazanavir, clopidogrel, cyclosporin, warfarin, acenocoumarol, carbamazepine, and antifungal agents, among others (171). A careful assessment of potential drug-drug interactions is also key to avert adverse events or unnecessary risks for therapy failure when using novel direct-acting antiviral agents such as sofosbuvir, ledipasvir, etc., where concomitant PPI use is advised against (172).
Furthermore, consideration of PPIs as simply gastric "protectors" with virtually no adverse effects has shot up their use for uncertain indications or symptoms not associated with acid hypersecretion. Also, their current low cost and the fact that may be easily obtained over-the-counter has favored their use and self-medication. Potentially serious side effects have been reported of late, which have been related to ongoing, long-term PPI use. These include symptomatic hypomagnesemia, higher risk for bone fracture, higher risk for infection (enteric infection or pneumonia), vitamin malabsorption, risk for potentially serious interactions (clopidogrel), primarily infectious complications in cirrhotic patients, and development of chronic renal disease, as was highlighted in a recent observational study (173). However, when the scientific evidence supporting potential PPI risks is analyzed, it is often based on observational studies or case reports endowed with obvious biases, including other likely causes for the reported side effects and inconsistent findings. Similarly, most reported studies exhibit blatant heterogeneity and inadequate control for potential confounders. The GRADE classification system for assessing the quality of evidence ascribes a low-quality score to evidence obtained from observational studies, which may be upgraded to moderate-quality for strong, highly consistent effects, with a dose-response relationship, and one plausible confounder. It is further considered that the observed effect of an intervention exhibits a weak association when its relative risk or odds ratio is smaller than 2, as is the case with most studies reviewed in the present paper. Furthermore, these effects usually concern polymedicated, frail elderly individuals with severe comorbidities, and immunosuppressed, malnourished patients, where PPI use may represent an additional risk marker rather than a causal association.
The association of PPI use with pneumonia, bone fracture, or a cardiovascular event should not be underestimated, as it may entail non-negligible morbidity and mortality rates. This fact must be borne in mind, and PPI use must be envisaged as a factor that, added to other factors, may trigger undesired effects.
To sum it all up, the evidence supporting an association between these adverse effects and long-term PPI use is difficult to interpret, lacks sufficient weight, and may be biased. Even so, we must remain cautious and alert in order to prevent such complications from arising in high-risk patients. Further studies and randomized trials are obviously needed, but higher-quality evidence is unlikely to result.
Presently, both the short-term and long-term benefits of PPI therapy exceed potential risks or side effects, provided clinical indication, dosage, and treatment duration are appropriate. Efforts should be made to avoid improper prescription, particularly in the polymedicated frail elderly and following hospitalization, and to inform patients regarding their treatment duration and how to keep clear from adverse effect-associated risk factors.
Conclusions and position statement
In summary, based on all the above, the statements considered by the SEPD as their positioning on PPIs and their potentially serious adverse effects include the following:
1. Overall, PPIs may be considered a safe drug class with few, mostly mild adverse effects.
2. Consideration of PPIs as simply gastric "protectors" with virtually no side effects has shot up their use often with no clear indication or for symptoms not associated with acid hypersecretion.
3. The need to use PPIs only when indicated, for the necessary duration, at the minimum effective dose, always under medical prescription is underscored by the SEPD.
4. Several potentially serious adverse effects have been associated with continued, long-term PPI use, but the evidence supporting such association is difficult to interpret and of insufficient weight, and may also be biased in multiple cases.
5. However, adverse effects with non-negligible morbidity and mortality rates do exist, which makes it advisable to regularly monitor appropriate PPI prescription, particularly in at-risk situations.
Acknowledgments
We are grateful to the SEPD Executive Committee members who, not having been authors themselves, have contributed to this paper's institutional review and support: Fernando Carballo, Enrique Domínguez, Cecilio Santander, Javier Crespo, Alfredo Lucendo, Enrique Pérez-Cuadrado, José Luis Calleja, Fernando Azpiroz, Joaquín Hinojosa, José Miguel Esteban, Julio Iglesias, and Miguel Muñoz-Navas. We also thank Joaquín León for his help with the documentation and literature.
Conflicts of interest
The authors who sign this position statement do so on behalf of the Sociedad Española de Patología Digestiva (SEPD). Neither this Scientific Society, nor any of the members of its task force are related whatsoever to the companies that develop the PPI drugs mentioned in this document. Neither the SEPD nor the members of its task force have any financial interests in the companies that researched and distribute these PPIs; however, both the above-mentioned Society and its task force members maintain an ongoing relationship with said companies for the promotion of education, research, and improved clinical practice regarding the conditions associated with PPI use. Finally, both the SEPD and the signing authors declare that the initiative to set up a task force and develop a position statement emerged and was carried out in the absence of any formal or informal connection with the industry, which had no influence and no knowledge whatsoever regarding the task force composition and the preliminary, intermediate, and final contents of the statement before its effective publication in The Spanish Journal of Gastroenterology (Revista Española de Enfermedades Digestivas).
References
1. Robinson M, Horn J. Clinical pharmacology of proton pump inhibitors - What the practising physician needs to know. Drugs 2003;63:2739-54. [ Links ]
2. García del Pozo J. Estudio de utilización de antiulcerosos en España (2000-2008). Inf Ter Sist Nac Salud 2009;33:49-54. [ Links ]
3. Ministerio de Sanidad. Subgrupos ATC y Principios activos de mayor consumo en el Sistema Nacional de Salud en 2010. Inf Ter Sist Nac Salud 2010;35:124-8. [ Links ]
4. Ponce J, Esplugues JV. Rationalizing the use of PPIs: An unresolved matter. Rev Esp Enferm Dig 2013;105:121-4. DOI: 10.4321/S1130-01082013000300001. [ Links ]
5. Simo Minana J. Use of prescription drugs in Spain and Europe. Aten Primaria. 2012;44:335-47. [ Links ]
6. Zink DA, Pohlman M, Barnes M, et al. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005;21:1203-9. DOI: 10.1111/j.1365-2036.2005.02454.x. [ Links ]
7. Eid SM, Boueiz A, Paranji S, et al. Patterns and predictors of proton pump inhibitor overuse among academic and non-academic hospitalists. Intern Med 2010;49:2561-8. DOI: 10.2169/internalmedicine.49.4064. [ Links ]
8. Walker NM, McDonald J. An evaluation of the use of proton pump inhibitors. Pharm World 9. Sci 2001;23:116-7. [ Links ]
9. de Burgos Lunar C, Novo del Castillo S, Llorente Diaz E, et al. Study of prescription-indication of proton pump inhibitors. Rev Clin Esp 2006;206:266-70. [ Links ]
10. Martin-Echevarria E, Pereira Julia A, Torralba M, et al. Assessing the use of proton pump inhibitors in an internal medicine department. Rev Esp Enferm Dig 2008;100:76-81. [ Links ]
11. Batuwitage BT, Kingham JGC, Morgan NE, et al. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J 2007;83:66-8. DOI: 10.1136/pgmj.2006.051151. [ Links ]
12. Ahrens D, Chenot J-F, Behrens G, et al. Appropriateness of treatment recommendations for PPI in hospital discharge letters. Eur J Clin Pharmacol 2010;66:1265-71. DOI: 10.1007/s00228-010-0871-9. [ Links ]
13. Cahir C, Fahey T, Teeling M, et al. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Brit J Clin Pharmacol 2010;69:543-52. DOI: 10.1111/j.1365-2125.2010.03628.x. [ Links ]
14. Villamanan E, Ruano M, Lara C, et al. Reasons for initiation of proton pump inhibitor therapy for hospitalised patients and its impact on outpatient prescription in primary care. Rev Esp Enferm Dig 2015;107:652-8. [ Links ]
15. Ramirez E, Lei SH, Borobia AM, et al. Overuse of PPIs in patients at admission, during treatment, and at discharge in a tertiary Spanish hospital. Curr Clin Pharmacol 2010;5:288-97. [ Links ]
16. Ahrens D, Behrens G, Himmel W, et al. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract 2012; 66:767-73. DOI: 10.1111/j.1742-1241.2012.02973.x. [ Links ]
17. Ponce Romero M, Berenguer Lapuerta J. Current indications for proton pump inhibitors. Rev Clin Esp 2003;203:136-8. [ Links ]
18. Grupo de trabajo Sector Zaragoza I Salud. Empleo de los inhibidores de la bomba de protones en la prevención de gastropatías secundarias a fármacos. Guía de Práctica Clínica. Zaragoza: Guía Salud; 2012. [ Links ]
19. Product Characteristics. Spanish Agency of Medicines and Medical Devices. [ Links ]
20. Arroyo Villarino M, Lanas Arbeloa A. Gastroenteropatía por AINE. En: Ponce García J, editor. Asociación Española de Gastroenterología. Tratamiento de las enfermedades gastroenterológicas. Barcelona: Elsevier; 2011. p. 123-31. [ Links ]
21. Pérez Gisbert J, Martín Argila C. Úlcera péptica e infección por Helicobacter pylori. En: Ponce García J, editor. Asociación Española de Gastroenterología. Tratamiento de las enfermedades gastroenterológicas. Barcelona: Elsevier; 2011. p. 109-21. [ Links ]
22. Sung JJY, Barkun A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding - a randomized trial. Ann Intern Med 2009;150:455-64. DOI: 10.7326/0003-4819-150-7-200904070-00105. [ Links ]
23. Sachar H, Vaidya K, Laine L. Intermittent vs continuous proton pump inhibitor therapy for high-risk bleeding ulcers - a systematic review and meta-analysis. JAMA Intern Med 2014;174:1755-62. DOI: 10.1001/jamainternmed.2014.4056. [ Links ]
24. Yen H-H, Yang C-W, Su W-W, et al. Oral versus intravenous proton pump inhibitors in preventing re-bleeding for patients with peptic ulcer bleeding after successful endoscopic therapy. BMC Gastroenterol 2012;12:66. DOI: 10.1186/1471-230X-12-66. [ Links ]
25. Pérez-Gisbert J, Calvet X, Ferrandiz J, et al. Clinical practice guideline on the management of patients with dyspepsia. Update 2012. Gastroenterol Hepatol 2012;35:725.e1-38. [ Links ]
26. Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2012;10:1066-78. [ Links ]
27. Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:13-22.e1. DOI: 10.1016/j.cgh.2015.07.041. [ Links ]
28. Molina-Infante J, Bredenoord AJ, Cheng E, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 2015; (online first): pii: gutjnl-2015-310991. Accesible en: http://www.ncbi.nlm.nih.gov/pubmed/26685124 DOI: 10.1136/gutjnl-2015-310991. [ Links ]
29. Dominguez-Munoz JE, Iglesias-Garcia J, Iglesias-Rey M. Optimising the therapy of exocrine pancreatic insufficiency by the association of a proton pump inhibitor to enteric coated pancreatic extracts. Gut 2006;55:1056-7. DOI: 10.1136/gut.2006.094912. [ Links ]
30. Stabler SP. Vitamin B-12 Deficiency. N Engl J Med 2013;368:149-60. [ Links ]
31. Jung SB, Nagaraja V, Kapur A, et al. Association between vitamin B12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis. Intern Med J 2015;45:409-16. [ Links ]
32. Lam JR, Schneider JL, Zhao W, et al. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B-12 deficiency. JAMA 2013;310:2435-42. DOI: 10.1001/jama.2013.280490. [ Links ]
33. Dharmarajan TS, Kanagala MR, Murakonda P, et al. Do acid-lowering agents affect vitamin B12 status in older adults? J Am Med Dir Assoc 2008;9:162-7. DOI: 10.1016/j.jamda.2007.10.004. [ Links ]
34. Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B-12 deficiency in older adults. J Clin Epidemiol 2004;57:422-8. DOI: 10.1016/j.jclinepi.2003.08.015. [ Links ]
35. Force RW, Meeker AD, Cady PS, et al. Increased vitamin B-12 requirement associated with chronic acid suppression therapy. Ann Pharmacother 2003;37:490-3. [ Links ]
36. Marcuard SP, Albernaz L, Khazanie PG. Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12). Ann Intern Med 1994;120:211-5. DOI: 10.7326/0003-4819-120-3-199402010-00006. [ Links ]
37. Mitchell SL, Rockwood K. The association between antiulcer medication and initiation of cobalamin replacement in older persons. J Clin Epidemiol 2001;54:531-4. DOI: 10.1016/S0895-4356(00)00340-1. [ Links ]
38. Koop H. Review article: Metabolic consequences of long-term inhibition of acid secretion by omeprazole. Aliment Pharmacol Ther 1992;6:399-406. DOI: 10.1111/j.1365-2036.1992.tb00553.x. [ Links ]
39. Termanini B, Gibril F, Sutliff VE, et al. Effect of long-term gastric acid suppressive therapy on serum vitamin B-12 levels in patients with Zollinger-Ellison syndrome. Am J Med 1998;104:422-30. DOI: 10.1016/S0002-9343(98)00087-4. [ Links ]
40. Rozgony NR, Fang C, Kuczmarski MF, et al. Vitamin B(12) deficiency is linked with long-term use of proton pump inhibitors in institutionalized older adults: could a cyanocobalamin nasal spray be beneficial? J Nutr Elder 2010;29:87-99. DOI: 10.1080/01639360903574734. [ Links ]
41. Koop H, Bachem MG. Serum iron, ferritin, and vitamin B12 during prolonged omeprazole therapy. J Clin Gastroenterol 1992;14:288-92. DOI: 10.1097/00004836-199206000-00005. [ Links ]
42. Schenk BE, Festen HPM, Kuipers EJ, et al. Effect of short- and long-term treatment with omeprazole on the absorption and serum levels of cobalamin. Aliment Pharmacol Ther 1996;10:541-5. DOI: 10.1046/j.1365-2036.1996.27169000.x. [ Links ]
43. Den Elzen WPJ, Groeneveld Y, De Ruijter W, et al. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment Pharmacol Ther 2008;27:491-7. DOI: 10.1111/j.1365-2036.2008.03601.x. [ Links ]
44. ter Heide H, Hendriks HJE, Heijmans H, et al. Are children with cystic fibrosis who are treated with a proton-pump inhibitor at risk for vitamin B-12 deficiency? J Pediatr Gastroenterol Nutr 2001;33:342-5. DOI: 10.1097/00005176-200109000-00023. [ Links ]
45. Tolia V, Boyer K. Long-term proton pump inhibitor use in children: A retrospective review of safety. Dig Dis Sci 2008;53:385-93. DOI: 10.1007/s10620-007-9880-7. [ Links ]
46. Hirschowitz BI, Worthington J, Mohnen J. Vitamin B12 deficiency in hypersecretors during long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther 2008;27:1110-21. DOI: 10.1111/j.1365-2036.2008.03658.x. [ Links ]
47. Long AN, Atwell CL, Yoo W, et al. Vitamin B-12 deficiency associated with concomitant metformin and proton pump inhibitor use. Diabetes Care 2012;35:E84-E. DOI: 10.2337/dc12-0980. [ Links ]
48. Attwood SE, Ell C, Galmiche JP, et al. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: data from the SOPRAN and LOTUS studies. Aliment Pharmacol Ther 2015;41:1162-74. DOI: 10.1111/apt.13194. [ Links ]
49. Bellou A, AimoneGastin I, DeKorwin JD, et al. Cobalamin deficiency with megaloblastic anaemia in one patient under long-term omeprazole therapy. J Intern Med 1996;240:161-4. DOI: 10.1046/j.1365-2796.1996.20846000.x. [ Links ]
50. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015;265:419-28. DOI: 10.1007/s00406-014-0554-0. [ Links ]
51. Rajabally YA, Jacob S. Neuropathy associated with lansoprazole treatment. Muscle Nerve 2005;31:124-5. DOI: 10.1002/mus.20155. [ Links ]
52. Sellapah S. An unusual side effect of omeprazole: case report. Br J Gen Pract 1990;40:389. [ Links ]
53. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int 2013;83:692-9. DOI: 10.1038/ki.2012.452. [ Links ]
54. Sumukadas D, McMurdo MET, Habicht D. Proton pump inhibitors are associated with lower magnesium levels in older people with chronic kidney disease. J Am Geriatr Soc 2012;60:392-3. DOI: 10.1111/j.1532-5415.2011.03808.x. [ Links ]
55. Gau J-T, Yang Y-X, Chen R, et al. Uses of proton pump inhibitors and hypomagnesemia. Pharmacoepidemiol Drug Saf 2012;21:553-9. DOI: 10.1002/pds.3224. [ Links ]
56. Sharara AI, Chalhoub JM, Hammoud N, et al. Low prevalence of hypomagnesemia in long-term recipients of proton pump inhibitors in a managed care cohort. Clin Gastroenterol Hepatol 2016;14:317-21. DOI: 10.1016/j.cgh.2015.10.012. [ Links ]
57. Chen J, Yuan YC, Leontiadis GI, et al. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol 2012;46:93-114. DOI: 10.1097/MCG.0b013e3182333820. [ Links ]
58. Epstein M, McGrath S, Law F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med 2006;355:1834-6. DOI: 10.1056/NEJMc066308. [ Links ]
59. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Riesgo de hipomagnesemia asociado a los medicamentos inhibidores de la bomba de protones (IBP). Ref. MUH (FV), 27/2011. Accesible en: http://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2011/docs/NI-MUH_27-2011.pdf. (visitado 23/12/15). [ Links ]
60. FDA Drug Safety Communication. Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. Washington, DC: US. Food and Drug Administration; 2011. Accesible en: http://www.fda.gov/Drugs/DrugSafety/ucm213206.htm. [ Links ]
61. Administration TG. Risk of hypomagnesaemia with proton pump inhibitors. Medicines Safety Update Vol 2, Number 3. Australian Prescriber 2011;34:81. Accesible en: https://www.tga.gov.au/publication-issue/medicines-safety-update-vol-2-no-3-june-2011. [ Links ]
62. Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: A systematic review and meta-analysis. PLoS One 2014;9:e112558. DOI: 10.1371/journal.pone.0112558. [ Links ]
63. Luk CP, Parsons R, Lee YP, et al. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother 2013;47:773-80. DOI: 10.1345/aph.1R556. [ Links ]
64. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail 2015;37:1237-41. DOI: 10.3109/0886022X.2015.1057800. [ Links ]
65. Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H-2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006;79:76-83. DOI: 10.1007/s00223-006-0021-7. [ Links ]
66. Yang Y-X, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-53. DOI: 10.1001/jama.296.24.2947. [ Links ]
67. Targownik LE, Lix LM, Metge CJ, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. Can Med Assoc J 2008;179:319-26. DOI: 10.1503/cmaj.071330. [ Links ]
68. Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008;28:951-9. DOI: 10.1592/phco.28.8.951. [ Links ]
69. Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008;83:251-9. DOI: 10.1007/s00223-008-9170-1. [ Links ]
70. Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009;84:13-9. DOI: 10.1007/s00223-008-9188-4. [ Links ]
71. Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010;139:93-101. DOI: 10.1053/j.gastro.2010.03.055. [ Links ]
72. Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women results from the women's health initiative. Arch Intern Med 2010;170:765-71. DOI: 10.1001/archinternmed.2010.94. [ Links ]
73. Pouwels S, Lalmohamed A, Souverein P, et al. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporos Int 2011;22:903-10. DOI: 10.1007/s00198-010-1337-8. [ Links ]
74. Reyes C, Formiga F, Coderch M, et al. Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone 2013;52:557-61. DOI: 10.1016/j.bone.2012.09.028. [ Links ]
75. Cea Soriano L, Ruigomez A, Johansson S, et al. Study of the association between hip fracture and acid-suppressive drug use in a UK primary care setting. Pharmacotherapy 2014;34:570-81. DOI: 10.1002/phar.1410. [ Links ]
76. Ding J, Heller DA, Ahern FM, et al. The relationship between proton pump inhibitor adherence and fracture risk in the elderly. Calcif Tissue Int 2014;94:597-607. DOI: 10.1007/s00223-014-9855-6. [ Links ]
77. van der Hoorn MMC, Tett SE, de Vries OJ, et al. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: A prospective cohort study. Bone 2015;81:675-82. DOI: 10.1016/j.bone.2015.08.024. [ Links ]
78. Freedberg DE, Haynes K, Denburg MR, et al. Use of proton pump inhibitors is associated with fractures in young adults: a population-based study. Osteoporos Int 2015; 26: 2501-7. DOI: 10.1007/s00198-015-3168-0. [ Links ]
79. Khalili H, Huang ES, Jacobson BC, et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. Br Med J 2012;344. DOI: 10.1136/bmj.e372. [ Links ]
80. Adams AL, Black MH, Zhang JL, et al. Proton-pump inhibitor use and hip fractures in men: a population-based case-control study. Ann Epidemiol 2014;24:286-90. [ Links ]
81. Lewis JR, Barre D, Zhu K, et al. Long-Term proton pump inhibitor therapy and falls and fractures in elderly women: A prospective cohort study. J Bone Miner Res 2014;29:2489-97. DOI: 10.1002/jbmr.2279. [ Links ]
82. Moberg LME, Nilsson PM, Samsioe G, et al. Use of proton pump inhibitors (PPI) and history of earlier fracture are independent risk factors for fracture in postmenopausal women. The 83. WHILA study. Maturitas 2014;78:310-5. DOI: 10.1016/j.maturitas.2014.05.019. [ Links ]
83. Chiu HF, Huang YW, Chang CC, et al. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf 2010;19:1131-6. DOI: 10.1002/pds.2026. [ Links ]
84. Fraser LA, Leslie WD, Targownik LE, et al. The effect of proton pump inhibitors on fracture risk: report from the Canadian Multicenter Osteoporosis Study. Osteoporos Int 2013;24:1161-8. [ Links ]
85. Abrahamsen B, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med 2011;171:998-1004. DOI: 10.1001/archinternmed.2011.20. [ Links ]
86. de Vries F, Cooper AL, Cockle SM, et al. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporos Int 2009;20:1989-98. DOI: 10.1007/s00198-009-0891-4. [ Links ]
87. Itoh S, Sekino Y, Shinomiya K-i, et al. The effects of risedronate administered in combination with a proton pump inhibitor for the treatment of osteoporosis. J Bone Miner Metab 2013;31:206-11. DOI: 10.1007/s00774-012-0406-9. [ Links ]
88. Lee J, Youn K, Choi NK, et al. A population-based case-control study: proton pump inhibition and risk of hip fracture by use of bisphosphonate. J Gastroenterol 2013;48:1016-22. DOI: 10.1007/s00535-012-0722-9. [ Links ]
89. Eom C-S, Park SM, Myung S-K, et al. Use of acid-suppressive drugs and risk of fracture: A meta-analysis of observational studies. Ann Fam Med 2011;9:257-67. DOI: 10.1370/afm.1243. [ Links ]
90. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol 2011;106:1209-18. DOI: 10.1038/ajg.2011.113. [ Links ]
91. Yu EW, Bauer SR, Bain PA, et al. Proton pump inhibitors and risk of fractures: A meta-analysis of 11 international studies. Am J Med 2011;124:519-26. [ Links ]
92. Yang S-D, Chen Q, Wei H-K, et al. Bone fracture and the interaction between bisphosphonates and proton pump inhibitors: a meta-analysis. Int J Clin Exp Med 2015; 8:4899-910. [ Links ]
93. Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int 2015. Accesible en: http://www.ncbi.nlm.nih.gov/pubmed/26462494. [ Links ]
94. Cumming RG. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging 1998;12:43-53. DOI: 10.2165/00002512-199812010-00005. [ Links ]
95. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med 2009;169:1952-60. DOI: 10.1001/archinternmed.2009.357. [ Links ]
96. Prieto-Alhambra D, Pages-Castella A, Wallace G, et al. Predictors of fracture while on treatment with oral bisphosphonates: A population-based cohort study. J Bone Miner Res 2014;29:268-74. DOI: 10.1002/jbmr.2011. [ Links ]
97. O'Connell MB, Madden DM, Murray AM, et al. Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. Am J Med 2005;118:778-81. DOI: 10.1016/j.amjmed.2005.02.007. [ Links ]
98. Wright MJ, Sullivan RR, Gaffney-Stomberg E, et al. Inhibiting gastric acid production does not affect intestinal calcium absorption in young, healthy individuals: A randomized, crossover, controlled clinical trial. J Bone Miner Res 2010;25:2205-11. DOI: 10.1002/jbmr.108. [ Links ]
99. Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: A population-based from the Canadian Multicentre Osteoporosis Study (CaMos). Am J Gastroenterol 2012;107:1361-9. DOI: 10.1038/ajg.2012.200. [ Links ]
100. Lau YT, Ahmed NN. Fracture risk and bone mineral density reduction associated with proton pump inhibitors. Pharmacotherapy 2012;32:67-79. DOI: 10.1002/PHAR.1007. [ Links ]
101. Solomon DH, Diem SJ, Ruppert K, et al. Bone mineral density changes among women initiating proton pump inhibitors or H2 receptor antagonists: A SWAN Cohort Study. J Bone Miner Res 2015;30:232-9. DOI: 10.1002/jbmr.2344. [ Links ]
102. Jo Y, Park E, Ahn SB, et al. A proton pump inhibitor's effect on bone metabolism mediated by osteoclast action in old age: A prospective randomized study. Gut Liver 2015;9:607-14. DOI: 10.5009/gnl14135. [ Links ]
103. Moayyedi P, Yuan Y, Leontiadis G, et al. Canadian Association of Gastroenterology position statement: Hip fracture and proton pump inhibitor therapy - a 2013 update. Can J Gastroenterol 2013;27:593-5. DOI: 10.1155/2013/321379. [ Links ]
104. Leontiadis GI, Moayyedi P. Proton pump inhibitors and risk of bone fractures. Curr Treat Options Gastroenterol 2014;12:414-23. DOI: 10.1007/s11938-014-0030-y. [ Links ]
105. Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007;102:2047-56. DOI: 10.1111/j.1572-0241.2007.01275.x. [ Links ]
106. Freeman R, Dabrera G, Lane C, et al. Association between use of proton pump inhibitors and non-typhoidal salmonellosis identified following investigation into an outbreak of Salmonella 107. Mikawasima in the UK, 2013. Epidemiol Infect 2015:1-8. DOI: 10.1017/S0950268815002332. [ Links ]
107. Wu H-H, Chen Y-T, Shih C-J, et al. Association between recent use of proton pump inhibitors and nontyphoid salmonellosis: A nested case-control study. Clin Infect Dis 2014;59:1554-8. DOI: 10.1093/cid/ciu628. [ Links ]
108. García Rodríguez LA, Ruigomez A, Panes J. Use of acid-suppressing drugs and the risk of bacterial gastroenteritis. Clin Gastroenterol Hepatol 2007;5:1418-23. [ Links ]
109. Kwok CS, Arthur AK, Anibueze CI, et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: Meta-analysis. Am J Gastroenterol 2012;107:1011-9. DOI: 10.1038/ajg.2012.108. [ Links ]
110. Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am J Gastroenterol 2012;107:1001-10. DOI: 10.1038/ajg.2012.179. [ Links ]
111. Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol 2012;10:225-33. DOI: 10.1016/j.cgh.2011.09.030. [ Links ]
112. McDonald EG, Milligan J, Frenette C, et al. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med 2015;175:784-91. DOI: 10.1001/jamainternmed.2015.42. [ Links ]
113. FDA Drug Safety Communication. Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). Washington, DC: US. Food and Drug Administration; 2012. Accesible en: http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm. [ Links ]
114. Laheij RJF, Sturkenboom M, Hassing RJ, et al. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004;292:1955-60. DOI: 10.1001/jama.292.16.1955. [ Links ]
115. Gulmez SE, Holm A, Frederiksen H, et al. Use of proton pump inhibitors and the risk of community-acquired pneumonia - A population-based case-control study. Arch Intern Med 2007;167:950-5. DOI: 10.1001/archinte.167.9.950. [ Links ]
116. Sarkar M, Hennessy S, Yang Y-X. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008;149:391-8. [ Links ]
117. Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010;31:1165-77. DOI: 10.1111/j.1365-2036.2010.04284.x. [ Links ]
118. Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol 2012;5:337-45. DOI: 10.1586/ecp.12.20. [ Links ]
119. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One 2015;10:e0128004. DOI: 10.1371/journal.pone.0128004. [ Links ]
120. Eom CS, Jeon CY, Lim JW, et al. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 2011;183:310-9. DOI: 10.1503/cmaj.092129. [ Links ]
121. Jena AB, Sun E, Goldman DP. Confounding in the association of proton pump inhibitor use with risk of community-acquired pneumonia. J Gen Intern Med 2013;28:223-30. DOI: 10.1007/s11606-012-2211-5. [ Links ]
122. Filion KB, Chateau D, Targownik LE, et al. Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: replicated cohort studies with meta-analysis. Gut 2014;63:552-8. DOI: 10.1136/gutjnl-2013-304738. [ Links ]
123. Kazui M, Nishiya Y, Ishizuka T, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 2010;38:92-9. DOI: 10.1124/dmd.109.029132. [ Links ]
124. Simon N, Finzi J, Cayla G, et al. Omeprazole, pantoprazole, and CYP2C19 effects on clopidogrel pharmacokinetic-pharmacodynamic relationships in stable coronary artery disease patients. Eur J Clin Pharmacol 2015;71:1059-66. DOI: 10.1007/s00228-015-1882-3. [ Links ]
125. Norgard NB, Mathews KD, Wall GC. Drug-drug interaction between clopidogrel and the proton pump inhibitors. Ann Pharmacother 2009;43:1266-74. DOI: 10.1345/aph.1M051. [ Links ]
126. Gilard M, Arnaud B, Cornily J-C, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin. J Am Coll Cardiol 2008;51:256-60. DOI: 10.1016/j.jacc.2007.06.064. [ Links ]
127. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909-17. [ Links ]
128. O'Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009;374:989-97. DOI: 10.1016/S0140-6736(09)61525-7. [ Links ]
129. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor insights from the platelet inhibition and patient outcomes trial. Circulation 2012;125:978-86. DOI: 10.1161/CIRCULATIONAHA.111.032912. [ Links ]
130. Siller-Matula JM, Jilma B, Schroer K, et al. Effect of proton pump inhibitors on clinical outcome in patients treated with clopidogrel: a systematic review and meta-analysis. J Thromb Haemost 2010;8:2624-41. DOI: 10.1111/j.1538-7836.2010.04049.x. [ Links ]
131. Hulot J-S, Collet J-P, Silvain J, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration a systematic meta-analysis. J Am Coll Cardiol 2010;56:134-43. DOI: 10.1016/j.jacc.2009.12.071. [ Links ]
132. Kwok CS, Loke YK. Meta-analysis: the effects of proton pump inhibitors on cardiovascular events and mortality in patients receiving clopidogrel. Aliment Pharmacol Ther 2010;31:810-23. DOI: 10.1111/j.1365-2036.2010.04247.x. [ Links ]
133. Kwok CS, Jeevanantham V, Dawn B, et al. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: Meta-analysis. Int J Cardiol 2013;167:965-74. DOI: 10.1016/j.ijcard.2012.03.085. [ Links ]
134. European Medicines Agency. Public statement: Interaction between clopidogrel and proton pump inhibitors. 2009; (29 may). Accesible en: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/11/WC500014409.pdf (visitado 23/12/15). [ Links ]
135. European Medicines Agency. Public statement: Interaction between clopidogrel and proton pump inhibitors (update). 2010; (17 mar). Accesible en: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2010/03/WC500076346.pdf (visitado 23/12/15). [ Links ]
136. Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole (27 oct). 2010. Accesible en: http://www.fda.gov/Drugs/DrugSafety/ucm231161.htm. (visitado 23/12/15). [ Links ]
137. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Interacción de clopidogrel con los inhibidores de la bomba de protones: actualización de la información y recomendaciones de uso (Nota informativa). 2010. Accesible en: http://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2010/docs/NI_2010-04_clopidogrel.pdf. (visitado 23/12/15). [ Links ]
138. Bell AD, Roussin A, Cartier R, et al. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines Executive Summary. Can J Cardiol 2011;27:208-21. DOI: 10.1016/j.cjca.2010.12.033. [ Links ]
139. Agewall S, Cattaneo M, Collet JP, et al. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur Heart J 2013;34:1708-13,13a-13b. DOI: 10.1093/eurheartj/eht042. [ Links ]
140. Shah NH, LePendu P, Bauer-Mehren A, et al. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One 2015;10. DOI: 10.1371/journal.pone.0124653. [ Links ]
141. Lundell L, Miettinen P, Myrvold HE, et al. Comparison of outcomes twelve years after antireflux surgery or omeprazole maintenance therapy for reflux esophagitis. Clin Gastroenterol Hepatol 2009;7:1292-8. DOI: 10.1016/j.cgh.2009.05.021. [ Links ]
142. Cardoso RN, Benjo AM, DiNicolantonio JJ, et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis. Open Heart 2015;2:e000248-e. DOI: 10.1136/openhrt-2015-000248. [ Links ]
143. Sherwood MW, Melloni C, Jones WS, et al. Individual proton pump inhibitors and outcomes in patients with coronary artery disease on dual antiplatelet therapy: A systematic review. J Am Heart Assoc 2015;4. DOI: 10.1161/JAHA.115.002245. [ Links ]
144. Weisz G, Smilowitz NR, Kirtane AJ, et al. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: The ADAPT-DES Study. Circ Cardiovasc Interv 2015;8. [ Links ]
145. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: Insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J 2015;170:683-94.e3. DOI: 10.1016/j.ahj.2015.05.017. [ Links ]
146. Hsieh C-F, Huang W-F, Chiang Y-T, et al. Effects of clopidogrel and proton pump inhibitors on cardiovascular events in patients with type 2 diabetes mellitus after drug-eluting stent implantation: A Nationwide Cohort Study. PLoS One 2015;10:e0135915. DOI: 10.1371/journal.pone.0135915. [ Links ]
147. Focks JJ, Brouwer MA, van Oijen MG, et al. Concomitant use of clopidogrel and proton pump inhibitors: impact on platelet function and clinical outcome- a systematic review. Heart 2013;99:520-7. DOI: 10.1136/heartjnl-2012-302371. [ Links ]
148. Melloni C, Washam JB, Jones WS, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes 2015;8:47-55. DOI: 10.1161/CIRCOUTCOMES. 114.001177. [ Links ]
149. Berger PB. Should proton pump inhibitors be withheld from patients taking clopidogrel? The issue that has been giving me heartburn! Circ Cardiovasc Qual Outcomes 2015;8:6-7. DOI: 10.1161/CIRCOUTCOMES.114.001586. [ Links ]
150. Herzig SJ, Howell MD, Ngo LH, et al. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120-8. DOI: 10.1001/jama.2009.722. [ Links ]
151. Linsky A, Gupta K, Lawler EV, et al. Proton pump inhibitors and risk for recurrent clostridium difficile infection. Arch Intern Med 2010;170:772-8. DOI: 10.1001/archinternmed.2010.73. [ Links ]
152. Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009;104:1130-4. DOI: 10.1038/ajg.2009.80. [ Links ]
153. Xu HB, Wang HD, Li CH, et al. Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res 2015;14:7490-501. DOI: 10.4238/2015.July.3.25. [ Links ]
154. Campbell MS, Obstein K, Reddy KR, et al. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008;53:394-8. DOI: 10.1007/s10620-007-9899-9. [ Links ]
155. Trikudanathan G, Israel J, Cappa J, et al. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract 2011;65(6):674-8. DOI: 10.1111/j.1742-1241.2011.02650.x. [ Links ]
156. Choi EJ, Lee HJ, Kim KO, et al. Association between acid suppressive therapy and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Scand J Gastroenterol 2011;46:616-20. DOI: 10.3109/00365521.2011.551891. [ Links ]
157. Goel GA, Deshpande A, Lopez R, et al. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol 2012;10:422-7. DOI: 10.1016/j.cgh.2011.11.019. [ Links ]
158. Deshpande A, Pasupuleti V, Thota P, et al. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: A meta-analysis. J Gastroenterol Hepatol 2013;28:235-42. DOI: 10.1111/jgh.12065. [ Links ]
159. de Vos M, De Vroey B, Garcia BG, et al. Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int 2013;33:1316-23. DOI: 10.1111/liv.12210. [ Links ]
160. Miura K, Tanaka A, Yamamoto T, et al. Proton pump inhibitor use is associated with spontaneous bacterial peritonitis in patients with liver cirrhosis. Intern Med 2014;53:1037-42. DOI: 10.2169/internalmedicine.53.2021. [ Links ]
161. Ratelle M, Perreault S, Villeneuve JP, et al. Association between proton pump inhibitor use and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Can J Gastroenterol Hepatol 2014;28:330-4. DOI: 10.1155/2014/751921. [ Links ]
162. Min YW, Lim KS, Min BH, et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther 2014;40:695-704. DOI: 10.1111/apt.12875. [ Links ]
163. Mandorfer M, Bota S, Schwabl P, et al. Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PLoS One 2014;9: e110503. DOI: 10.1371/journal.pone .0110503. [ Links ]
164. O'Leary JG, Reddy KR, Wong F, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:753-9.e1-2. [ Links ]
165. Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: A multicenter prospective study. J Hepatol 2015;62:1056-60. DOI: 10.1016/j.jhep.2014.11.036. [ Links ]
166. Chang SS, Lai CC, Lee MT, et al. Risk of spontaneous bacterial peritonitis associated with gastric Acid suppression. Medicine (Baltimore) 2015;94:e944. DOI: 10.1097/MD.0000000000000944. [ Links ]
167. Kwon JH, Koh S-J, Kim W, et al. Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol 2014;29:775-81. DOI: 10.1111/jgh.12426. [ Links ]
168. Merli M, Lucidi C, Di Gregorio V, et al. The chronic use of beta-blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int 2015;35:362-9. DOI: 10.1111/liv.12593. [ Links ]
169. van Vlerken LG, Huisman EJ, van Hoek B, et al. Bacterial infections in cirrhosis: role of proton pump inhibitors and intestinal permeability. European Journal of Clinical Investigation 2012;42:760-7. DOI: 10.1111/j.1365-2362.2011.02643.x. [ Links ]
170. Dultz G, Piiper A, Zeuzem S, et al. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther 2015;41:459-66. DOI: 10.1111/apt.13061. [ Links ]
171. Emre Y, Mesut S, Aylin Y, et al. Adverse drug reactions due to drug-drug interactions with proton pump inhibitors: assessment of systematic reviews with AMSTAR method. Expert Opin Drug Saf 2015. Accesible en: http://www.ncbi.nlm.nih.gov/pubmed/26635063. [ Links ]
172. Honer Zu Siederdissen C, Maasoumy B, Marra F, et al. Drug-Drug Interactions With Novel All Oral Interferon-Free Antiviral Agents in a Large Real-World Cohort. Clin Infect Dis 2015. Accesible en: http://www.ncbi.nlm.nih.gov/pubmed/26611779. [ Links ]
173. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016;176:238-46. [ Links ]
![]() Correspondence:
Correspondence:
Cristóbal de la Coba.
Coordinador Comité de Excelencia Clínica.
Sociedad Española de Patología Digestiva
e-mail: delacoba76@hotmail.com
Received: 28-01-2015
Accepted: 12-02-2015











 text in
text in