Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.5 Madrid may. 2016
Liver failure posthepatectomy and biliary fistula: multidisciplinar treatment. Case report
Fallo hepático posthepatectomía y fístula biliar: manejo multidisciplinar a propósito de un caso
Javier Calleja-Kempin1, Arturo Colón-Rodríguez1, Pedro Machado-Liendo1, Agustín-Acevedo2, Jorge Martín-Gi3, Teresa Sánchez-Rodríguez3 and Laura Zorrilla-Matilla1
1 General Surgery Department. Hospital San Francisco de Asís. Madrid, Spain.
2 Pathology Department. Hospital Quirón. Madrid, Spain.
3 General Surgery Department. Hospital Quirón San José. Madrid, Spain
Case Report
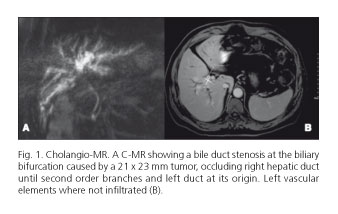
A 59-years-old patient with a good general condition and no associated risk factors for any disease, was referred to surgery because of right abdominal pain and pruritus with laboratory biochemical values of 2.5 mg/dl bilirubin, 400 GGT U/l, FA 350 U/l, CA 19.9 437 U/ml, CEA 5 ng/ml and a cholangio-MR showing a bile duct stenosis at the biliary bifurcation caused by a 21 x 23 mm tumor, occluding right hepatic duct until second order branches and left duct at its origin. Left vascular elements were not infiltrated (Fig. 1). Liver volumetry showed a 40% hepatic remnant (737 cc, index > 0.8 adjusted to weight and corporal surface). After these studies, portal embolization was not considered. Neither bile duct drainage was decided because biochemical values and good performance status of the patient.
Once Bismuth IV perihilar cholangiocarcinoma was established, the patient underwent a right hepatectomy extended to segment I and IVb. Every right vasculo-biliar elements and left bile duct were transected above the tumor, obtaining a free macroscopic and microscopic margin. A complete common bile duct resection plus hepatic hilum lymphadenectomy was achieved, expanding lymphadenectomy to common hepatic artery and celiac trunk. Liver hepatectomy was done by ultrasonic suction (CUSA) without Pringle maneuver at any moment. A Roux-Y jejunostomy was performed for biliary reconstruction. A good patient hemodynamic status was maintained during the procedure, needing only two units of erythrocyte concentrated.
Pathology specimen was reported as perihilar cholangiocarcinoma moderately differentiated (gr2), mass pattern (no periductal), without surgical margin infiltration. There were no vascular or perineural invasion, and all 17 lymphatic nodules were negative (0/17), being a T3 N0 stage.
After surgery, patient was admitted to UCI hemodynamically well stabilized, removing tracheal tube 10 hours after. First lab values were: Prothrombin activity 35%; bilirubin 6.6 mg/dl; AST 839 U/l; CPK 1118 U/l with glucose and lactate in normal values. Abdominal drains were as high as 2.5 l (lightly hematic ascites) and diuresis was relatively low (1.1 l/24 h).
After first 24 hours a posthepatectomy liver failure was considered (PHLF), starting somatostatine treatment at initial dose of 50 µg/8 h and increasing to 250 mcg/8 h during following days. Also we used propanolol at starting dosage of 100 mg/12 h (portal flow control) and vitamin K at 150 µg /12 h (coagulopathy).
At the same time patient was treated as a hepatic insufficiency, with low protein, low salt diet and lactulose enemas. Although encephalopathy was not observed, bilirubinemia and ascites progressed. Every two days -paracentesis were needed, obtaining a biliary staining in some of them.
At the 17th postop day, with not enough response to treatment (bilirubin 15 mg/dl), a splenic artery embolization was considered in order to diminish splanchnic flow. Successful embolization was achieved by interventional radiology (with coils). After this procedure, bilirubin drops down to 8 mg/dl, maintaining low synthetic function (prothrombin activity < 50%) and ascites.
At 25th postop day, patient was transferred to UCI because of dyspnea, hypotension and renal failure. Abdominal CT showed bile collections in the upper abdomen. A new surgery was done for abdominal lavage and drains setting. Biliary fistula was not evidenced during the procedure and portal pressure was measured at the level of ileocolic vein (18 mmHg). Patient status improves after laparotomy and cultures of abdominal liquid were positive for Staphylococcus epididermidis and faecium. An antibiotic treatment was established with linezolid, daptomicine, meropenem and fluconazol (partly prophylactic). Biliary fistula was completely drained by Jackson-Pratt drains as shown in HIDA Tc-99m scan, with a very low bile flow through hepatic-jejunal anastomosis (Fig. 2).
At 31st day, the patient developed a new sepsis, with partial infarction of the spleen at CT-scan. A new laparotomy was considered for splenectomy. After splenectomy, the hepatic-jejunal anastomosis was done again over a trans-anastomotic trans-jejunal multiperforated silicone drain and drawing out through abdominal wall, used for bile measurement. Furthermore, a liver wedge biopsy was obtained, showing microvesicular steatosis and hemorrhagic focus in the parenchyma, all these findings compatible with SFSS-PHLF (1) (Fig. 3).
Biliary fistula volume progressively increases as liver regeneration improved, deciding bile reinfusion through jejunal catheter to maintain an enterohepatic bile circulation with better liver function and, by hence, a better clinical condition. At the 80th postoperative day, the patient was discharged with propanolol 50 mg/12 h and furosemide 40 mg/12 h as all treatment. Bile production was reinfused during night at domicile using an enteral-feeding pump (by spouse).
Six months after first surgical procedure, an almost complete liver regeneration was observed by CT-scan (Fig. 4) and measured bile flow of 700 cc, a new surgical procedure for biliary reconstruction was considered. A complex dissection of a posterior bile fistula trajectory was achieved, using this for "bilio-jejunal" anastomosis using previous roux-y, tying at the same time the left hepatic duct with low bile flow (measured by HIDA Tc-99 scan). Patient did well postoperatively; being discharged in a good general condition with good liver function and an almost complete compensatory liver hypertrophy measured by CT-scan (Fig. 4).
Discussion
In the last two decades, morbimortality after major hepatectomy has decreased by a better patient preoperative evaluation and better surgical and anesthetic techniques, nevertheless, if the liver has some kind of pathology, liver regeneration could be impaired ending in a so called PHLF, similar to a SSFS, described by Edmond et al. in living donor transplantation (2). Both syndromes are characterized by cholestasis, coagulopathy, refractory ascites and finally encephalopathy, and have a dismal prognosis with a high mortality (3). In these entities, an excessive splanchnic flow with high portal pressure represents the same physiopathology mechanism, with clear pathological findings in the liver parenchyma as centrolobullillar necrosis and cholestasis, associated to liver insufficiency and poor compensatory regeneration of the liver (1). In the case described above, PHLF diagnosis was considered from the beginning according to clinical, laboratory and, finally, biopsy findings.
Preoperative liver function and functional reserve after hepatectomy can be measured by functional test as green indociane clearing test (4) and liver volumetry by CT-scan, adjusted to weight and corporal surface (5). Considered minimal liver remnant to achieve a posthepatectomy good liver function is 25% for healthy livers and 40% in case of some kind of liver pathology, taking into account this principle, calculated residual volume by CT of our patient was about 40 % with no risk factors as advanced age or diabetes (6), not considering preoperative portal embolization to increase liver volume. However, cholestasis could contribute to PHLF development, although there is no agreement among the authors. In this sense, Cherqui et al. did not find increased morbimortality after major hepatectomy in jaundiced patients comparing to bile-drained patients (7). In the same way, Sennath et al. (2002), showed an increment of morbidity and hospital stay in liver and pancreatic surgery of patients previously drained, with same mortality in both groups (8). As commented before, biliary drainage was discarded in the present case because of low bilirubin levels, in order to avoid complications of the transhepatic drainage.
High portal blood venous flow has gained a central role in the pathogenesis of SFSS and PHLF in a decreased intrahepatic portal venous tree capacity, developing the so-called hepatic "arterial buffer response". In a regular condition, there is an intimal relationship between portal and arterial flow, in this way, after a reduction of portal blood flow, an increment of arterial flow is activated. After a major hepatectomy (> 50-60%), not only portal flow is too high for liver remnant, also arterial flow increases impairing compensatory liver regeneration (9). Liver regeneration is also impaired by cholestasis developed in the portal hypertension syndrome, with increasing levels of HFG and VEGF (10).
Then, the main principle of PHLF treatment is to decrease portal flow and pressure by pharmacological, radiological or surgical methods, to avoid liver damage and improve regeneration. According to the International Study Group in Liver Surgery, the patient developed a liver failure grade C, needing both medical treatment and interventional techniques (2). Pharmacologically, somatostatine and propranolol were use for portal blood flow blood control. Somatostatine is a peptide used for treatment of esophagic variceal bleeding in portal hypertension by splanchnic blood reduction without systemic hemodynamic effects, avoiding endoteline-1 effects on portal flow (11). Another drug with similar effects in splanchnic flow and regeneration is terlipresine (12). In our case increasing dosage of somatostatine was introduced until clinical stabilization.
Splenic artery embolization and splenectomy have been tried in SSFS as in PHLF with good results and improved survival (13,14). However, splenectomy contributes to infections as observed in the case. In this sense, PHLF can be worsened by bacterial and fungal infections (15), then, an antibiotic prophylaxis is needed after splenectomy, as show in a clinical trial (16).
Vitamin K (daily basis), and plasma and factor VII (occasionally) were used successfully in the treatment of coagulopathy. Coagulation parameters and bilirubinemia were the main values for liver function evaluation. Bilirubin figures have a relevant clinical importance because when total bilirubin increases to 7 mg/dL after major resection postoperative mortality increases too (17). To complete the clinical syndrome follow-up, ascites and encephalopathy have to be treated, ascites with diuretics and paracentesis and encephalopathy with diet and antibiotics, although encephalopathy only shows in late stages of the course, auguring a bad prognosis.
The biliary fistula was relevant in this case under a clinical point of view. Bile volume drained by abdominal Jackson-Pratt drainage, served us as a marker of liver function, and its reinfusion through a jejunal catheter was a key factor for an enterohepatic bile circulation with improvement of metabolic and liver function.
Both PHLF and SFSS are major cause of mortality after hepatectomy and living donor transplantation. In conclusion, liver failure has to be recognized as soon as possible and treated aggressively in a multidisciplinary way, with different specialists collaborations for pharmacological treatment and interventions, to handle liver function, portal flow and complications.
References
1. Demetris AJ, Kelly DM, Eghtesad B, et al. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol 2006;30:986-93. DOI: 10.1097/00000478-200608000-00009. [ Links ]
2. Emond JC, Renz JF, Ferrell LD, et al. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg 1996;224:544-52-discussion 552-4. DOI: 10.1097/00000658-199610000-00012. [ Links ]
3. Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. DOI: 10.1016/j.surg.2010.10.001. [ Links ]
4. Imamura H, Sano K, Sugawara Y, et al. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg 2005;12:16-22. DOI: 10.1007/s00534-004-0965-9. [ Links ]
5. Vauthey J. Body surface area and body weight predict total liver volume in Western adults. Liver Transplantation 2002;8:233-40. DOI: 10.1053/jlts.2002.31654. [ Links ]
6. Hammond JS, Guha IN, Beckingham IJ, et al. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188-200. DOI: 10.1002/bjs.7630. [ Links ]
7. Cherqui D, Benoist S, Malassagne B, et al. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg 2000;135:302-8. DOI: 10.1001/archsurg.135.3.302. [ Links ]
8. Sewnath ME, Karsten TM, Prins MH, et al. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg 2002;236:17-27. DOI: 10.1097/00000658-200207000-00005. [ Links ]
9. Eipel C. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. WJG 2010;16:6046. DOI: 10.3748/wjg.v16.i48.6046. [ Links ]
10. Yagi S, Iida T, Hori T, Taniguchi K, et al. Optimal portal venous circulation for liver graft function after living-donor liver transplantation. Transplantation 2006;81:373-8. DOI: 10.1097/01.tp.0000198122.15235.a7. [ Links ]
11. Xu X, Man K, Zheng SS, et al. Attenuation of acute phase shear stress by somatostatin improves small-for-size liver graft survival. Liver Transpl 2006;12:621-7. DOI: 10.1002/lt.20630. [ Links ]
12. Fahrner R, Patsenker E, de Gottardi A, et al. Elevated liver regeneration in response to pharmacological reduction of elevated portal venous pressure by terlipressin after partial hepatectomy. Transplantation 2014;97:892-900. DOI: 10.1097/TP.0000000000000045. [ Links ]
13. Yoshizumi T, Taketomi A, Soejima Y, et al. The beneficial role of simultaneous splenectomy in living donor liver transplantation in patients with small-for-size graft. Transpl Int 2008;21:833-42. DOI: 10.1111/j.1432-2277.2008.00678.x. [ Links ]
14. Umeda Y, Yagi T, Sadamori H, et al. Preoperative proximal splenic artery embolization: A safe and efficacious portal decompression technique that improves the outcome of live donor liver transplantation. Transpl Int 2007;20:947-55. DOI: 10.1111/j.1432-2277.2007.00513.x. [ Links ]
15. Schindl MJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005;54:289-96. DOI: 10.1136/gut.2004.046524. [ Links ]
16. Wu CC, Yeh DC, Lin MC, et al. Prospective randomized trial of systemic antibiotics in patients undergoing liver resection. Br J Surg 1998;85:489-93. DOI: 10.1046/j.1365-2168.1998.00606.x. [ Links ]
17. Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. ACS 2007;204:854-62-discussion 862-4. DOI: 10.1016/j.jamcollsurg.2006.12.032. [ Links ]
![]() Correspondence:
Correspondence:
Javier Calleja Kempin.
General Surgery Department.
Hospital San Francisco de Asís.
C/ Joaquín Costa, 28.
28002 Madrid, Spain
e-mail: jcallejakempin@msn.com
Received: 25-02-2015
Accepted: 07-04-2015











 texto en
texto en