My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 n.6 Madrid Jun. 2016
Effectiveness of three interventions to improve participation in colorectal cancer screening
Jesús López-Torres-Hidalgo1, Joseba Rabanales-Sotos2, María José Simarro-Herráez3, Jaime López-Torres-López4, Monchi Campos-Rosa5 and M.a Ángeles López-Verdejo6
1 Centro de Salud de Albacete Zona IV (Servicio de Salud de Castilla-La Mancha/SESCAM) and Facultad de Medicina de Albacete. Universidad de Castilla-La Mancha (UCLM). Albacete, Spain.
2 Facultad de Enfermería de Cuenca. Universidad de Castilla-La Mancha. Cuenca, Spain.
3 Centro de Salud de Villarrobledo. Servicio de Salud de Castilla-La Mancha. Albacete, Spain.
4 Department of Anesthesiology. Hospital Universitario e Instituto Politécnico La Fe. Valencia, Spain.
5 Department of Pharmacy. Complejo Hospitalario Universitario de Albacete. Albacete, Spain.
6 Department of Occupational Health. Complejo Hospitalario Universitario de Albacete. Albacete, Spain
This study was funded by an Oncology Research grant (Ayuda a la Investigación en Oncología 2010) from the Asociación Española Contra el Cáncer (Albacete Provincial Board) (resolution dated 29 November 2010).
ABSTRACT
Background and objective: Participation in colorectal cancer (CRC) screening varies widely among different countries and different socio-demographic groups. Our objective was to assess the effectiveness of three primary-care interventions to increase CRC screening participation among persons over the age of 50 years and to identify the health and socio-demographic-related factors that determine greater participation.
Methods: We conducted a randomized experimental study with only one post-test control group. A total of 1,690 subjects were randomly distributed into four groups: written briefing; telephone briefing; an invitation to attend a group meeting; and no briefing. Subjects were evaluated 2 years post-intervention, with the outcome variable being participation in CRC screening.
Results: A total of 1,129 subjects were interviewed. Within the groups, homogeneity was tested in terms of socio-demographic characteristics and health-related variables. The proportion of subjects who participated in screening was: 15.4% in the written information group (95% confidence interval [CI]: 11.2-19.7); 28.8% in the telephone information group (95% CI: 23.6-33.9); 8.1% in the face-to-face information group (95% CI: 4.5-11.7); and 5.9% in the control group (95% CI: 2.9-9.0), with this difference proving statistically significant (p < 0.001). Logistic regression showed that only interventions based on written or telephone briefing were effective. Apart from type of intervention, number of reported health problems and place of residence remained in the regression model.
Conclusions: Both written and telephone information can serve to improve participation in CRC screening. This preventive activity could be optimized by means of simple interventions coming within the scope of primary health-care professionals.
Key words: Colorectal cancer. Cancer screening. Primary health care.
Introduction
Colorectal cancer (CRC) is a disease suitable for screening, since it poses a health problem of great magnitude and there are tests which are capable of detecting it in the initial stages, when treatment is most effective. The scientific community supports the advisability of screening, which has a favorable benefit-risk balance (1), and there is wide consensus on the need to raise the awareness of the population, health professionals and health authorities alike as to the importance of preventing this disease (2). For some considerable time, the European Union, the US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care have been recommending that population-based CRC screening be implemented (3).
Studies show that the fecal occult blood test (FOBT) can detect 60-85% of tumors and colonoscopy with polypectomy can reduce mortality by 60-90% (4). Thanks to early diagnosis and treatment, the incidence of this disease has decreased over the last two decades (5).
Many countries have already implemented population-based CRC screening programs and many more are on the point of doing so. Although there are a number of screening modalities, the choice of test is influenced by the cost, available resources, and acceptance of the test by part of the population. At present, no test has matched FOBT's availability and effectiveness, which is supported by clinical trials (6).
Spain's National Health System Cancer Strategy includes the proposal for population-based CRC screening to be performed on men and women from the age of 50 years upwards every 2 years, using the FOBT (7). This strategy also envisages progressive implementation and sets a coverage target of 50% by 2015 (8). Indeed, eleven Spanish regional authorities had initiated screening programs by 2014, and the remainder either implemented pilot programs or had plans to introduce screening during the course of that year. Furthermore, a number of scientific societies, patients' associations and non-governmental organizations have decided to join forces in the "Alliance for the Prevention of Colorectal Cancer in Spain" (9).
As with many other preventive activities, primary health-care professionals play a fundamental role in CRC prevention, by disseminating primary prevention measures, urging screening of the average-risk population, identifying high-risk individuals by means of the appropriate personal and family histories, and working alongside specialized care in the tasks of managing and following up individuals with specific colorectal lesions (10). Nevertheless, participation in these programs is not only low, but also lower than that registered in other cancer prevention programs (2). Among the reasons for such low participation are (2) the perception that the examinations involved are painful and unpleasant, and population's ignorance (shared by some health professionals) of the importance of CRC prevention programs. It is therefore both advisable and pertinent to ascertain which interventions are effective when it comes to increase participation in CRC screening.
Accordingly, this study had a dual aim: firstly, to assess the effectiveness of three primary-care interventions, consisting in providing information in writing, by telephone and on a face-to-face basis, to increase participation in CRC screening among men and women over the age of 50 years, in a geographical area where a population-based screening program has not yet been implemented; and secondly, to identify the health and socio-demographic-related factors that determine greater screening participation.
Material and methods
Setting and participants
A randomized experimental study with only one post-test control group was conducted on men and women aged 50 to 74 years affiliated to the Albacete Health Area (south-east Spain), across the period of January 2012-December 2014.
To select participants, we used two-stage sampling in which the primary units were 36 basic health zones in the Albacete Health Area (8 of which were randomly selected), and the secondary units were persons aged 50 to 74 years belonging to these zones. In each of these basic health zones, subjects were selected by simple random sampling. Participants were then randomly distributed into 4 groups (Fig. 1): the first group received a letter containing written information about CRC screening; the second group received this information via a telephone call made by health personnel; the third group was invited to receive information by attending a group meeting at their health center; and the fourth group received no information and was regarded as the control group. Based on an expected frequency of participation in CRC screening of 8% in the control group and 15% in the group subjected to the least effective intervention over a 2-year period, a statistical power of 80% and an alpha error of 5%, the required sample size in each group was 325 participants (total 1,300). This sample size was increased by 30% to offset foreseeable non-responses (total 1,690).
Variables and measurement
In each of these forms of communication (written, telephonic or face-to-face), the intervention consisted in providing detailed information on current preventive recommendations and screening method, and suggesting that recipients attend their health center for the purpose of undergoing FOBT. These recommendations included using FOB determination by means of an immunological test every two years as a CRC screening test for all persons aged 50 to 74 years. In all cases, a motivational strategy was pursued, with it being recognized that it was the individual's responsibility to care for his/her own health. Written information was developed specifically for the study. Information by telephone was provided by three nurses, who had previously attended two training sessions. In the case of the face-to-face information group, groups of a maximum of 12 were formed and sessions, taught by nurses, lasted approximately 45 minutes.
Once the interventions had concluded, subjects were evaluated after a period of two years: to this end, they were asked to come to their respective health centers to answer a questionnaire which contained socio-demographic variables, health-related variables and variables related to participation in CRC screening, whether in the preceding two years or at some time in their lives. The interviews were conducted by health professionals. After being requested to attend their health centers, the following subjects were excluded: those with a history of colorectal cancer; and those with severe sensory impairment or low intellectual performance of a level insufficient to collaborate in the study. During the interview, participants were required to give their written informed consent. The study was approved by the Clinical Research Ethics Committee of the Albacete Health Area.
The outcome variable was participation or non-participation in CRC screening in the 2-year period preceding the interview. Other variables covered by the interview were: history of participation in other cancer screening programs in the case of women (cervical and breast cancer); personal history of cancer and history of cancer among first-degree relatives; diseases or health problems classified by the International Classification of Primary Care (WONCA ICPC-2 classification); presence of comorbidity assessed using the Charlson Index (11); self-perceived health (very good, good, fair, poor or very poor); health status assessed with the EUROQOL-5D questionnaire (12); level of cancer worry as measured by the Cancer Worry Scale (13); degree of satisfaction with and use made of health-care services (ordinal scale); and socio-demographic characteristics (sex, age, marital status, educational level, and rural or urban area of residence).
Statistical analysis
The replies were entered into a database, processed and analyzed. The variables of interest and potentially confounding variables were compared in all groups, to ascertain whether, despite the use of a random allocation system, there was homogeneity among the groups for the baseline values of the study variables. The incidence of the outcome variable in the respective groups was then described and compared to that of the control group (comparison of proportions using the Chi-square test), with an alpha error of 5% in two-sided hypotheses. Other intergroup comparisons were performed using the Student's t-test (comparison of means in two groups) or McNemar test (comparison between the proportions of women who had participated in CRC screening and cervical and breast cancer screening). Lastly, to analyze the effect of interventions on screening participation we constructed a logistic regression model for explanatory purposes, identifying the existence of possible interactions between screening participation and socio-demographic characteristics, perceived health, presence of comorbidity and cancer worry. The dependent variable was participation in screening at any time during the intervening period between the interventions and the subsequent evaluation performed at the end of 2 years. Interventions were introduced into the model as dummy variables, taking the control group as reference. The statistical significance of each of the coefficients was evaluated using the Wald test. All analyses were performed using the SPSS v. 19.0 statistical software program.
Results
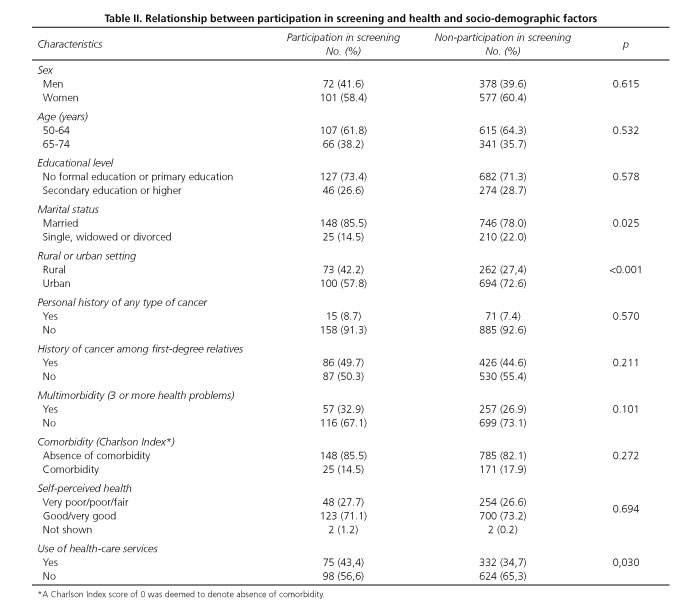
Interviews were held with a total of 1,129 men and women aged 50 to 74 years, belonging to the basic health zones of La Roda, Socovos, Elche de la Sierra, Riopar and the city of Albacete Zones III, IV, VB and VII. The proportion of women was 60.1% and the mean age of all the participants was 61.4 years (standard deviation [SD]: 6.6). Participants' distribution in the study groups is shown in figure 1. In the written information group 298 subjects could be interviewed (70.6%), in the telephone information group 316 (74.7%), in the face-to-face information group 246 (58.3%) and in the control group 269 (63.6%). In the face-to-face information group, in no case did attendance at such sessions exceed 29.6%. Table I shows the characteristics of these groups, with homogeneity among them being tested in terms of socio-demographic characteristics and health-related variables. Among the study participants there was predominance of subjects who were married or in a stable relationship (79.2%), subjects with no formal education or only primary education (71.7%), and subjects from urban settings (70.3%). Although most subjects enjoyed good or very good health (73.2%), 27.8% reported multimorbidity (3 or more health problems).
Among women there was a statistically significant association (p < 0.001) between participation in colorectal cancer screening and participation in both breast cancer screening (97.0% of women who had undergone FOBT had also participated in breast cancer screening, versus 89.8% of women with no FOBT) and cervical cancer screening (70.3% of women who had undergone FOBT had also participated in cervical cancer screening, versus 50.9% of women with no FOBT).
No statistically significant differences were observed in terms of age and sex between subjects who had undergone FOBT testing in the two years preceding the interview and those who had not done so (Table II). In the sample as a whole, no relationship was observed between participation in colorectal cancer screening and the existence of personal history of any type of cancer or history of any type of cancer among first-degree relatives. Similarly, there was no difference between participants and non-participants regarding the status of the dimensions assessed with the EuroQol 5D questionnaire (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Likewise, the comparison of the mean score obtained in perceived health status, assessed on a scale of 0 to 100, showed no statistically significant difference between participants and non-participants. However, participants in screening registered a significantly higher score (p = 0.005) on the Cancer Worry Scale (10.1 ± 3.8 SD vs 9.2 ± 3.1 SD).
In terms of satisfaction with health-care services, screening participants did not display a higher level of satisfaction with their general practitioners or specialized services. In the case of health-care service use, however, screening participants were observed to register a higher frequency of visits to the general practitioner, with the proportion of persons who had made such visits in the preceding month being significantly higher (p = 0.03) among the screening participants (43.4% vs. 34.7%).
The proportion of men and women that had participated in colorectal cancer screening during the two years preceding the interview was 15.4% in the written information group (95% CI: 11.2-19.7), 28.8% in the telephone information group (95% CI: 23.6- 33.9), 8.1% in the face-to-face information group (95% CI: 4.5-11.7) and 5.9% in the control group (95% CI: 2.9-9.0), with the difference proving statistically significant (p < 0.001). Of the subjects who attended the face-to-face information groups (73 persons), 13 (17.8%) underwent FOBTs. The 875 participants who had never participated in screening cited the following reasons for their decision: enjoyment of good health (32.8%); oversight or lack of time (24.5%); ignorance of the test or lack of information (18.4%); fear of an abnormal result or the need for colonoscopy (14.4%); and other reasons (9.9%). In 14 instances (1.6%), subjects reported not having undergone the test as a result of being advised against doing so by their physician.
Logistic regression showed that only interventions based on written or telephone briefing were effective vis-à-vis the control group (Table III). The model also includes variables that showed a statistically significant association: number of reported health problems, rural or urban setting, marital status, cancer worry and use of health-care services. Apart from type of intervention, number of reported health problems and place of residence (rural or urban setting) remained in the regression model as independent variables associated with participation in screening. Persons with more health problems and those from rural settings were those who had participated most during the two years preceding the interview. In this model, no statistically significant interactions were observed between type of intervention and socio-demographic variables, perceived health status or cancer worry, thus indicating that these were not modifying factors of the interventions undertaken.
Discussion
The results show that in four uniform groups of men and women over the age of 50 years, interventions that provide written or telephone information about CRC screening are effective in terms of increasing participation in such screening. Face-to-face information may also be effective but percentage attendance at information meetings was very low, which doubtlessly prevented better results from being obtained among those belonging to this group. It was also possible to establish that screening participation was higher in the case of rural settings and subjects who reported more health problems. Furthermore, among women there was an association between participation in CRC screening and participation in breast and cervical cancer screening.
Among the study's limitations, mention should be made of limited response indices, particularly in the face-to-face information group, with the non-response rate proving higher than initially envisaged. Although homogeneity of responders in all the groups was established, the characteristics of the non-participants in each of these could not be compared due to lack of data. Another potential limitation was a possible contamination bias between groups, which could have reduced the differences among them. Lastly, a further limitation lay in possible differences in the behavior and motivation of health professionals who informed the study subjects about screening, which might have influenced the effectiveness of the interventions.
At present, cancer screening's most important limitation is acknowledged to be population participation. In order to achieve the benefits to be expected from implementing a screening program, participation is an essential aspect, in that it has a direct impact on the effectiveness of such screening. Low population participation in these programs acts as a bar to obtaining positive cost-effectiveness results, even in cases in which there is sufficient evidence regarding the efficacy of screening, as in CRC. In England, where FOBT has been available to persons over the age of 50 years since 2006, a coverage of 54% has been reported, with the reasons for this low participation not being clear (14). Our results show that, among other things, reasons for low participation include enjoying good health and failure to perceive the risk of developing CRC, insufficient knowledge of screening and the disease, and fear of an abnormal result. Other studies have also reported other circumstances, such as absence of symptoms of the disease or not receiving a letter of invitation (15). In contrast, better knowledge and keener awareness as to the importance of the disease are associated with greater screening participation (14). Some of these circumstances could, however, be changed by supplying appropriate information about the screening test and the disease, with the manner of furnishing such information being crucial, as seen from our results. Furthermore, it has been noted that the reasons cited for not participating in screening tend to differ according to sex, age and social class (15).
"Lack of recommendation by doctors" is one of the barriers to screening participation described in the literature (16). A noteworthy finding in our results was that on some occasions patients were advised against having the screening test by their own physicians, quite possibly due to the latter being unaware of the recommendations contained in current clinical practice guidelines. Although the proportion of professionals who frequently recommend screening to the average-risk population is low in our area (17), a previous study enabled us to establish that most primary health-care professionals consider screening as effective and acceptable for their patients (17). Many studies have consistently shown that health professional's recommendation is the single most influential factor for participation in screening (4).
Some factors have been associated with CRC screening participation (18,19), though results tend to differ in accordance with geographical settings. Our results show that subjects belonging to a rural setting and subjects with a greater number of health problems were those who participated most in screening. In general, there is a wide overall variation among countries in terms of CRC screening participation (19). Studies such as this can help to ascertain the differences in each area or region and thus contribute to improving adherence to screening programs.
To date, different interventions have been studied for the purpose of ascertaining their impact on adherence to CRC screening. Written communication and electronic communication adapted to the characteristics of the population have both yielded good results (20-27), especially among immigrant population (28,29), and even in the workplace (30). Similarly, good results have also been obtained by interventions that combine written information with new technologies, such as text messages, which have a low cost (31). In contrast, some educational interventions (32) and others based on the combination of written and telephone information (33) have not yielded the expected results. In our case, telephone calls not only enabled information to be provided, but also made it possible to resolve any doubts about the test and the disease, something that may well have contributed to achieving greater effectiveness. A review of studies targeted at ascertaining the effectiveness of interventions to improve participation in CRC screening concludes that the most successful interventions are those that target individuals or communities, envisage barriers to screening, contain messages tailored to the population, use different forms of communication, and are maintained over time (34).
In conclusion, both written and telephone information can significantly improve CRC screening participation among subjects aged over 50 years, something that could be useful for optimizing population screening once implemented. The results of this study suggest that an explanation about the disease and the procedure for its early detection helps individuals to accept the screening program. Current preventive strategies could be optimized through simple interventions that come within the scope of health professionals.
References
1. Marzo-Castillejo M, Mascort Roca J, Pastor Rodríguez-Moñino A, et al. ¿Estamos convencidos de nuestro papel en la prevención y detección precoz del cáncer colorrectal? Aten Primaria 2012;44: 303-5. [ Links ]
2. Andreu García M, Marzo M, Mascort J, et al. Prevention of colorectal cancer. Gastroenterol Hepatol 2009;32:137-9. DOI: 10.1016/j.gastrohep.2008.12.001. [ Links ]
3. Pignone M, Rich M, Teutsch SM, et al. Screening for colorectal cancer in adults at average risk. A summary of the evidence. Ann Intern Med 2002;137:132-41. DOI: 10.7326/0003-4819-137-2-200207160-00015. [ Links ]
4. Spruce LR, Sanford JT. An intervention to change the approach to colorectal cancer screening in primary care. J Am Acad Nurse Pract 2012;24:167-74. DOI: 10.1111/j.1745-7599.2012.00714.x. [ Links ]
5. Short MW, Layton MC, Teer BN, et al. Colorectal cancer screening and surveillance. Am Fam Physician 2015;91:93-100. [ Links ]
6. Benton SC, Seaman HE, Halloran SP. Fecal occult blood testing for colorectal cancer screening: The past or the future. Curr Gastroenterol Rep 2015;17:428. DOI: 10.1007/s11894-015-0428-2. [ Links ]
7. Estrategia en Cáncer del Sistema Nacional de Salud. Ministerio de Sanidad y Política Social. Plan de Calidad para el Sistema Nacional de Salud. Madrid: Ministerio de Sanidad y Política Social; 2010. [ Links ]
8. Salas D. Colorectal cancer screening: Foundations for making progress in screening in Spain. Gac Sanit 2011;25:329-30. DOI: 10.1016/j.gaceta.2011.05.004. [ Links ]
9. Morillas JD, Castells A, Oriol I, et al. Alianza para la Prevención del Cáncer de Colon en España: un compromiso cívico con la sociedad. Gastroenterol Hepatol 2012;35:109-28. DOI: 10.1016/j.gastrohep.2012.01.002. [ Links ]
10. Bellas Beceiro B, Ferrándiz Santos J, Mascort Roca JJ, et al. Clinical practice guidelines for prevention of colorectal cancer: Towards an integral, integrated and coordinated approach. Aten Primaria 2004;34:451-3. DOI: 10.1016/S0212-6567(04)79528-1. [ Links ]
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373-83. DOI: 10.1016/0021-9681(87)90171-8. [ Links ]
12. EuroQol group. EuroQol - A new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. DOI: 10.1016/0168-8510(90)90421-9. [ Links ]
13. Cabrera E, Zabalegui A, Blanco I. Spanish version of the Cancer Worry Scale (CWS). Cross cultural adaptation and validity and reliability analysis. Med Clin (Barc) 2011;136:8-12. DOI: 10.1016/j.medcli.2010.04.015. [ Links ]
14. Gale CR, Deary IJ, Wardle J, et al. Cognitive ability and personality as predictors of participation in a national colorectal cancer screening program: The English Longitudinal Study of Ageing. J Epidemiol Community Health 2015;69.530-5. DOI: 10.1136/jech-2014-204888. [ Links ]
15. Molina-Barceló A, Salas-Trejo D, Peiró-Pérez R, et al. Reasons for participating in the Valencian Community Colorectal Cancer Screening Program by gender, age, and social class. Rev Esp Enferm Dig 2014;106:439-47. [ Links ]
16. Javadzade SH, Reisi M, Mostafavi F, et al. Barriers related to fecal occult blood test for colorectal cancer screening in moderate risk individuals. J Educ Health Promot 2014;3:120. DOI: 10.4103/2277-9531.145928. [ Links ]
17. López-Torres Hidalgo J, Simarro Herráez MJ, Rabanales Sotos J, et al. The attitudes of primary care providers towards screening for colorectal cancer. Rev Esp Enferm Dig 2013;105:272-8. DOI: 10.4321/S1130-01082013000500005. [ Links ]
18. Larkey LK, McClain D, Roe DJ, et al. Randomized controlled trial of storytelling compared to a personal risk tool intervention on colorectal cancer screening in low-income patients. Am J Health Promot 2015;30:e59-70. DOI: 10.4278/ajhp.131111-QUAN-572. [ Links ]
19. Decker KM, Singh H. Reducing inequities in colorectal cancer screening in North America. J Carcinog 2014;13:12. DOI: 10.4103/1477-3163.144576. [ Links ]
20. Resnicow K, Zhou Y, Hawley S, et al. Communication preference moderates the effect of a tailored intervention to increase colorectal cancer screening among African-Americans. Patient Educ Couns 2014;97:370-5. DOI: 10.1016/j.pec.2014.08.013. [ Links ]
21. Lee HY, Tran M, Jin SW, et al. Motivating underserved Vietnamese Americans to obtain colorectal cancer screening: Evaluation of a culturally tailored DVD intervention. Asian Pac J Cancer Prev 2014;15:1791-6. DOI: 10.7314/APJCP.2014.15.4.1791. [ Links ]
22. Lairson DR, Dicarlo M, Deshmuk AA, et al. Cost-effectiveness of a standard intervention versus a navigated intervention on colorectal cancer screening use in primary care. Cancer 2014;120:1042-9. DOI: 10.1002/cncr.28535. [ Links ]
23. Green BB, Wang CY, Anderson ML, et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: A randomized trial. Ann Intern Med 2013;158:301-11. DOI: 10.7326/0003-4819-158-5-201303050-00002. [ Links ]
24. Myers RE, Bittner-Fagan H, Daskalakis C, et al. A randomized controlled trial of a tailored navigation and a standard intervention in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev 2013;22:109-17. DOI: 10.1158/1055-9965.EPI-12-0701. [ Links ]
25. Fiscella K, Humiston S, Hendren S, et al. A multimodal intervention to promote mammography and colorectal cancer screening in a safety-net practice. J Natl Med Assoc 2011;103:762-8. DOI: 10.1016/S0027-9684(15)30417-X. [ Links ]
26. Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: Results of a pilot study. BMC Fam Pract 2009;10:37. DOI: 10.1186/1471-2296-10-37. [ Links ]
27. Shankaran V, McKoy JM, Dandade N, et al. Costs and cost-effectiveness of a low-intensity patient-directed intervention to promote colorectal cancer screening. J Clin Oncol 2007;25:5248-53. DOI: 10.1200/JCO.2007.13.4098. [ Links ]
28. Tu SP, Chun A, Yasui Y, et al. Adaptation of an evidence-based intervention to promote colorectal cancer screening: A quasi-experimental study. Implement Sci 2014;9:85. DOI: 10.1186/1748-5908-9-85. [ Links ]
29. Aragones A, Schwartz MD, Shah NR, et al. A randomized controlled trial of a multilevel intervention to increase colorectal cancer screening among Latino immigrants in a primary care facility. J Gen Intern Med 2010;25:564-7. DOI: 10.1007/s11606-010-1266-4. [ Links ]
30. McFall AM, Ryan JE, Hager P. Implementing a client reminder intervention for colorectal cancer screening at a health insurance worksite. Prev Chronic Dis 2014;11:E20. DOI: 10.5888/pcd11. 130276. [ Links ]
31. Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: A randomized clinical trial. JAMA Intern Med 2014;174:1235-41. DOI: 10.1001/jamainternmed.2014.2352. [ Links ]
32. Carney PA, Lee-Lin F, Mongoue-Tchokote S, et al. Improving colorectal cancer screening in Asian-Americans: Results of a randomized intervention study. Cancer 2014;120:1702-12. DOI: 10.1002/cncr.28640. [ Links ]
33. Leone LA, Reuland DS, Lewis CL, et al. Reach, usage, and effectiveness of a Medicaid patient navigator intervention to increase colorectal cancer screening, Cape Fear, North Carolina, 2011. Prev Chronic Dis 2013;10:E82. DOI: 10.5888/pcd10.120221. [ Links ]
34. Powe BD, Faulkenberry R, Harmond L. A review of intervention studies that seek to increase colorectal cancer screening among African-Americans. Am J Health Promot 2010;25:92-9. DOI: 10.4278/ajhp.080826-LIT-162. [ Links ]
![]() Correspondence:
Correspondence:
Jesús López-Torres-Hidalgo.
Centro de Salud Universitario Zona IV.
C/ Seminario, 4.
02006 Albacete, Spain
e-mail: jesusl@sescam.org
Received: 20-10-2015
Accepted: 04-04-2016