My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 n.8 Madrid Aug. 2016
https://dx.doi.org/10.17235/reed.2016.4183/2016
Hepatic preneoplasia induction in male Wistar rats: histological studies up to five months post treatment
Gerardo Bruno Pisani1, José Luis Valenti2 and Alejandra Beatriz Quintana1
1Department of Physiology. Morphology Area. Facultad de Ciencias Bioquímicas y Farmacéuticas. Universidad Nacional de Rosario. Rosario, Santa Fe. República Argentina.
2School of Medicine. Pathology Area. Universidad Abierta Interamericana. Rosario, Santa Fe. República Argentina
This study was supported by the secretary of Science, Technology and Innovation of Santa Fe, Scientific Project No. 2010-093-11, Argentina.
ABSTRACT
Background: Liver preneoplasia development in rats can be mimicked by an initiation-promotion model that induces the appearance of altered hepatocyte foci (FAH).
Aims: We compare two initiation-promotion models to evaluate the presence of FAH or additional hepatic pathologies in which other organs were affected up to five month post treatment.
Material and methods: FAH were induced in male adult Wistar rats with two doses of dietylnitrosamine (DEN, 150 mg/kg bw) followed by 4 doses per week (3 weeks) of 2-acetylaminofluorene (2-AAF, 20 mg/kg bw) or with one dose of DEN (200 mg/kg bw) followed by 2 doses per week (3 weeks) of 2-AAF. DEN 150, DEN 200 and control rats (received the vehicle of the drugs) groups were compared. Rats were euthanized immediately after the last dose of 2-AAF, at 3, 4 and 5 months (n = 3 for euthanasia times per group). Samples of livers, lungs, kidneys, pancreatic tissue and small bowel were processed for histological and immunohistochemical analysis.
Results: FAH persisted for 5 months in all livers of the DEN groups. Three months after withdrawal of 2-AAF, one rat from DEN 150 group developed fibrosis and 5 months after 2-AAF removal another rat from the same group presented a microscopic hyperplastic nodule. Only the lungs had damages compatible with lesions induced by gavage-related reflux in DEN groups.
Conclusion: We concluded that up to five month post treatments, FAH persisted in all the livers from DEN groups; livers from DEN 200 group showed no other hepatic lesions besides FAH, and only the lungs suffered pathological alterations in both treated groups.
Key words: Diethilnitrosamine. 2-acetylaminofluorene. Rat liver preneoplasia. Foci of altered hepatocytes. Reflux.
Introduction
Cancer development is known to be a multistep process. The concept of multi-stage carcinogenesis was first proposed by Berenblum and Schubik in 1948 and supported by later studies. Present day oncology recognizes three main phases: initiation, promotion and progression (1).
Neoplasia initiation is essentially irreversible changes in appropriate target somatic cells. Briefly, initiation involves one or more stable cellular changes arising spontaneously or induced by exposure to a carcinogen. This is considered to be the first step in carcinogenesis, where the cellular genome undergoes mutations, creating the potential for neoplastic development (2,3).
The transformed (initiated) cell can remain harmless, unless and until it is stimulated to undergo further proliferation, upsetting the cellular balance. The subsequent changes of an initiated cell leading to neoplastic transformation may involve more than one step and require repeated and prolonged exposures to promoting stimuli (4). During promotion, these cells expand by proliferation generating proliferative foci that resemble benign neoplasms which can regress to a seemingly normal tissue or slowly evolve to cancer (5).
At the progression state, successive changes take place in the neoplasm and give rise to increasingly malignant sub-populations (1).
It has been reported that DEN is a representative chemical carcinogen with the potential to cause tumors in various organs (6,7) and it is widely used as a chemical initiator in rodent models of experimental hepatocarcinogenesis (8). In rats, DEN influences the initiation stage of hepatic carcinogenesis by producing DNA base modifications and DNA strand-breaks that contribute to the development of putative preneoplastic focal lesions (6,9). Following the initiation stage, the administration of promoting agents, such as 2-acetylaminofluorene (2-AAF), causes selective enhancement of the proliferation of initiated hepatocytes over non-initiated cells (10).
Several models in rat liver have been developed to study multistage carcinogenesis, including the Solt-Farber resistant hepatocyte model (11). The system consists of: a) a single carcinogenic dose of DEN; b) short-term dietary exposure to 2-acethylaminofluorene (2-AAF), sufficient to suppress growth of virtually all normal hepatocytes; and c) partial hepatectomy to promote rapid growth of DEN-altered hepatocytes (11,12).
Álvarez et al. designed a 2-phase model (initiation-promotion) of liver preneoplasia in rats that eliminated the need for partial hepatectomy. In this model, rats were subjected to two necrogenic doses of DEN two weeks apart and four doses per week of 2-AAF by gavage for three weeks. Foci of altered hepatocytes (FAH) emerged in livers of treated rats at the end of the six-week treatment. At this time, a state of subclinical hepatic preneoplasia was observed (10). The initiation-promotion or 2-phase model of cancer mimics the early events of the latent period of human carcinogenesis (11). We simplified the model of Álvarez et al. (DEN 150 model) by reducing the frequency of chemical agent administration, and thus, the total dose of carcinogens given to experimental animals (DEN 200 model). In a previous study, we compared DEN 150 model with DEN 200 establishing that both protocols were good alternatives to induce liver preneoplasia in rats (13).
The results obtained compared these two alternatives to induce hepatic preneoplasia after promoter removal and up to five month post treatment; this may help researchers to decide which protocol causes less morphological damage in livers and in other organs.
The aims of this study were to compare two initiation-promotion models to evaluate the presence of FAH in rat livers, to investigate if additional hepatic pathologies were present and to study if other organs were affected by carcinogens up to five month post treatment.
Material and methods
Animals
Male adult Wistar rats (300-350 g body weight) were maintained two per cage with a constant 12 hs light/dark cycle under controlled temperature and humidity conditions. They had free access to tap water. They were fed with standard rat pellets ad libitum. All the experimental protocols were performed according to the NIH "Guide for the Care and Use of Laboratory Animals" (14) and approved by the "Guide for the Care and Use of Laboratory Animals Committee", Facultad de Ciencias Bioquímicas y Farmacéuticas, UNR, Resolution No 6109/012.
Experimental design
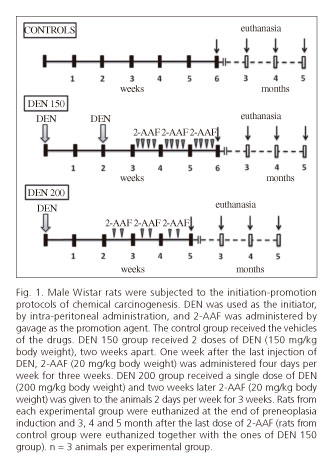
Animals were divided into 3 groups. Rats of group 1 were used as normal controls and received the vehicle of the drugs (n = 10). Rats from groups 2 and 3 were subjected to a 2-stage model of rat hepatocarcinogenesis (n = 12 per group). In group 2 (DEN 150 group), the initiation stage was performed by the administration of 2 intraperitoneal necrogenic doses of DEN (150 mg/kg body weight) two weeks apart. Administration of 2-AAF was performed one week after the last injection of DEN. The 2-AAF was dissolved in dimethyl sulfoxide and then suspended in corn oil to a final concentration of 8 mg/ml. Rats received 20 mg/kg body weight of 2-AAF by gavage for 4 consecutive days per week for 3 weeks (10). Animals from group 3 (DEN 200 group) were subjected to a 2-stage model of rat hepatocarcinogenesis adapted from the one of Álvarez et al. In this protocol, a unique dose of DEN (200 mg/kg body weight) was administrated intraperitoneally as an initiation stage, and two weeks later a dose of 20 mg/kg body weight of 2-AAF was given by gavage for 2 days (one day apart) per week for 3 weeks (13).
Rats were euthanized at the end of preneoplasia induction and 3, 4 and 5 month (n = 3 for each time of euthanasia per group) after the last dose of 2-AAF. Until euthanized animals received only tap water and were fed with standard rat pellets. Controls were euthanized simultaneously with rats of DEN 150 group (Fig. 1). Euthanizes were performed between 9 and 11 a.m. to avoid variations due to circadian rhythm.
Histological process
Samples of livers, lungs, pancreatic tissue, kidneys and small bowel were obtained from all animals, fixed in 10% v/v formaldehyde and histologically processed to be paraffin embedded. Sections of 4 µm thicknesses tissues were histological analyzed with hematoxylin and eosin, Masson's trichrome and Direct Red 80/picric acid.
Immunohistochemical studies
FAH were identified performing an immunohistochemical detection using an antibody against the placental form of rat glutathione S-transferase (rGST-P). This isozyme has been described as the best effective marker of hepatic preneoplasia in rats (15). Replicating cells were identified with an antibody that recognizes an endogenous marker of cell replication, proliferating cell nuclear antigen (PCNA) (16). Briefly, deparaffinized tissue sections were treated with 3% H2O2 in methanol for 10 min to remove endogenous peroxidase, and then they were microwaved in a 10 mM buffer citrate solution for 10 min at 96 oC to perform antigen retrieval. Normal serum was applied to the slides to block nonspecific binding and then, consecutive slides were incubated either with rabbit polyclonal antibody against rGST-P (Abcam®, catalogue AB106268, USA) diluted 1/250 at 4 oC overnight or anti-PCNA (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1/400 at room temperature for 2 hs. The slides were incubated with a biotinylated goat anti-rabbit secondary antibody and then with horseradish-peroxidase-conjugated streptavidin (HRP CytoScan Detection Kit, Cell Marque, catalogue CMD302, USA). Signals were detected with the DAB Sustrate Kit (Cell Marque, catalogue 957D-20, USA) followed by hematoxylin counterstaining (n = 3 per group).
Results
Histological and immunohistochemical findings
Only livers and lungs presented notorious histological alterations; other organs showed no morphological damages.
Livers
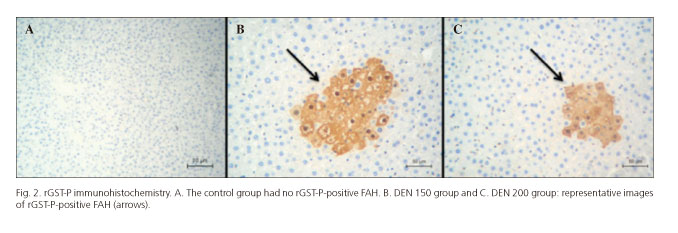
At the end of both treatments, rat livers from DEN 150 and DEN 200 groups presented rGST-P-positive FAH at all times of sacrifice. Controls livers had no FAH (Fig. 2).
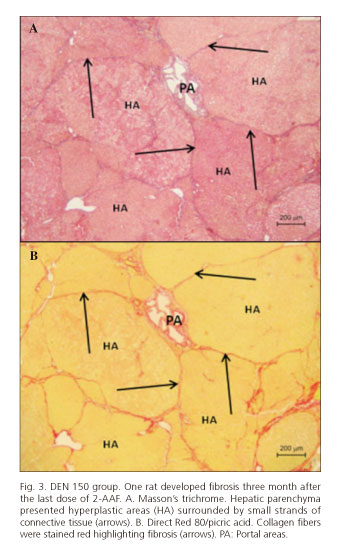
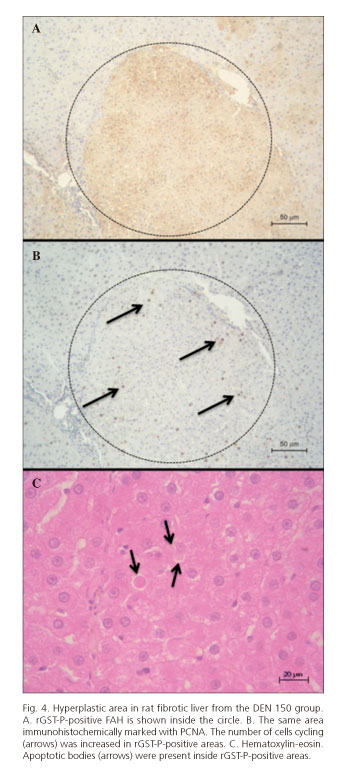
Immediately after the last dose of 2-AAF, animals from both experimental groups presented rGST-P-positive FAH and no other hepatic damage or diseases. After 3 months, only one rat from DEN 150 group developed liver fibrosis with a hepatic parenchyma constituted by hyperplastic areas surrounded by little strands of connective tissues that resembled micronodular cirrhosis in its initial stage. An increase of cell replication and apoptotic bodies was also found in hyperplastic areas (Figs. 3 and 4). The two other rats from this group and the ones from DEN 200 group only developed rGST-P-positive FAH.
After 4 months, all rats of both experimental groups showed only rGST-P-positive FAH and no other histological alterations.
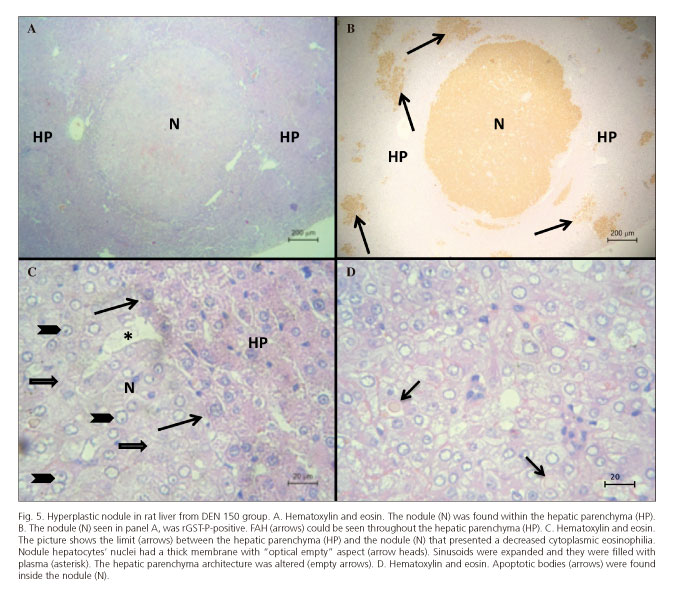
After 5 months only one rat from DEN 150 group had a large hyperplastic nodule together with rGST-P-positive FAH. The two other rats from this group and the ones from the DEN 200 group only showed rGST-P-positive FAH. The nodule observed showed a decreased cytoplasmic eosinophilia (Figs. 5A, C and D) which was positive for rGST-P immunostaining (Fig. 5B) with compressive limits. Nodule hepatocytes' nuclei had thick membrane with "optical empty" aspect. The nodule compressed the surrounding parenchyma with evidence of angiogenesis together with dilated sinusoids filled with plasma and the hepatocyte trabecular morphology was completely altered (Fig. 5C). Isolated apoptotic bodies were also found inside the nodule (Fig. 5D).
Lungs
Lungs of all rats from the treated groups at all times of sacrifice presented morphological alterations that could be induced by gavage-related reflux (17). Some bronchus showed pyogenic infiltrates and others presented large vacuolated macrophages inside. Many bronchial lumens were filled with abundant mucus. A general atelectasis could be observed, medial hyperplasia of the arteriolar vessels diminished the vascular lumen and few inflammatory foci infiltrated the alveolus. The control group did not show any morphological alterations (Fig. 6).
Discussion
We have shown that rGST-P-positive FAH were found in all rats from both DEN groups and that FAH persisted after withdrawal of the promoter (2-AAF) during a five month period in both treated groups. Persistent FAH has been reported by many authors (18,19).
After three months of 2-AAF removal, one rat from the DEN 150 group developed a widespread fibrosis and after five month a rat from the same experimental group presented a large rGST-P-positive hyperplastic nodule. Fibrosis was observed with Direct Red 80/picric acid, a specific stain for collagen (20). Hyperplasia was demonstrated with PCNA immunostaining, showing an increased number of cells cycling in rGST-P-positive areas.
Apoptotic bodies were found with hematoxylin-eosin staining in most rGST-P-positive areas (21). During regression of mitogen-induced hyperplasia, non-proliferating (aged) hepatocytes seemed to be preferred for apoptosis. Furthermore, studies on apoptosis in putative preneoplastic foci of rat liver have shown that foci cells exhibit about 10-fold higher rates of apoptosis than normal hepatocytes. It appears that both cell replication and cell death by apoptosis are regulated in concert to produce regression or growth of organs and that these basic mechanisms are still functioning in malignant tumors, although they are obviously out of balance (22).
The remainder of rats from both experimental groups developed only rGST-P-positive FAH. Rats from the DEN 150 group showed not only rGST-P-positive FAH but also fibrosis and a hepatic nodule at two different times of sacrifice. Possibly, these differences, compared with animals from the DEN 200 group, could be assigned to the higher doses of initiator and promoter used in the DEN 150 group that might induce additional hepatic diseases.
Lung alteration could be assigned to gavage-related reflux that can occur by mechanically induced reflux or spontaneous reflux. Mechanically induced reflux occurs directly after the gavage when withdrawing the tube from the animal and spontaneous reflux occurs not only directly after gavage, but also later, when the animal is back in its cage. The later reflux is related to the administration of a large volume by gavage (5-10 mL) (17). However, regarding gavage-related reflux and technical gavage errors, it is now considered that even very small amounts of the treated material, 20 µl, are able to induce serious irritation in the respiratory tract and mortality. Mechanically induced reflux is considered as the most likely cause of reflux in rats (23). In both compared protocols, 20 mg/kg body weight of 2-AAF was given to animals by gavage in a final volume of 0.5 mL; therefore, this volume was enough to induce respiratory alterations.
In many protocols the promoter is usually sprayed on food and given to rats with a subsequent partial hepatectomy or not (24,25). DEN 200 group uses fewer doses of 2-AAF per week that could be beneficial to diminish mechanically induced reflux, hence reducing pulmonary complications. However, rats from this experimental group presented morphological alterations in the lungs. Curiously, respiratory damages from both treated groups did not increase rat mortality or respiratory symptoms to consider terminating the experiments. Moreover, animals showed normal habits of hygiene, alimentation and mobility during the whole treatment, and none died or needed to be euthanized. It could be expected to observe morphological alterations in the lungs due to DMSO gavage-reflux. Nonetheless, no damage was found in the present study in the control group.
DEN 150 and DEN 200 two-phase or initiation-promotion models mimic the latent period of human hepatocarcinogenesis and are considered as useful tools to study the early stages of liver cancer. DEN 200 model was less aggressive for experimental animals, inducing only FAH and no other hepatic diseases; however, lungs suffered the same damage as in DEN 150 model animals. The comparison of histological effects of both models on liver and other organs allows us to choose the most useful methodology to be applied with the aim of resolving particular experimental objective.
Our study has the following limitations: first, although rGST-P is a frequently used and reliable marker for FAH in rat liver, it does not reflect the persistent FAH after withdrawal of the inducing compounds, that do not only increase in size but also change their cellular phenotype during progression as demonstrated in many studies by cytomorphological, cytochemical, microbiochemical and molecular methods (26). Second, it is necessary to analyze the FAH phenotype evolution through time in our experimental models. Third, to avoid respiratory complications due to gavage, the amount of toxic agent administrated and the irritancy and the viscosity of the test formulations, among others considerations, should be controlled (23).
In future studies the number and size of FAH should be established by quantitative morphometric approaches and statistical differences between times and groups regarding the sizes of FAH should be calculated. FAH cellular phenotype changes have to be studied and it would be necessary to improve the gavage technique in order to diminish respiratory complications.
We concluded that up to five month post treatment, rGST-P-positive FAH persisted in all livers from DEN groups; livers from the DEN 200 group showed no other hepatic lesions apart from rGST-P-positive FAH, and only the lungs suffered pathological alterations in both treated groups.
Acknowledgements
The authors acknowledge histotechnologist Alejandra Inés Martínez for her excellent technical assistance and Juan Pablo Parody, PhD, for digital image processing.
References
1. Devi PU. Basics of carcinogenesis. Health Adm 1998;17:16-24. [ Links ]
2. Weston A, Harris CC. Multistage carcinogenesis. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. Available from: http://www.ncbi.nlm.nih.gov/books/NBK13982/. [ Links ]
3. Cox R. Mechanisms of radiation oncogenesis. Int J Radiat Biol 1944;65:57-64. DOI: 10.1080/09553009414550081. [ Links ]
4. Dragan YP, Hully J, Crow R, et al. Incorporation of bromodeoxyuridine in glutathione S-transferase-positive hepatocyte during rat multistage hepatocarcionogenesis. Carcinogenesis 1994;15:1939-47. DOI: 10.1093/carcin/15.9.1939. [ Links ]
5. Farber E. Cancer development and its natural history. A cancer prevention perspective. Cancer 1988;62:1676-9. DOI: 10.1002/1097-0142(19881015)62:1+<1676::AID-CNCR2820621303>3.0.CO;2-1. [ Links ]
6. Shirakami Y, Gottesman ME, Blaner WS. Diethylnitrosamine-induced hepatocarcinogenesis is suppressed in lecithin:retinol acyltransferase-deficient mice primarily through retinoid actions immediately after carcinogen administration. Carcinogenesis 2012;33:268-74. DOI: 10.1093/carcin/bgr275. [ Links ]
7. Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: Bioactivation, DEN-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 1996;71:57-81. DOI: 10.1016/0163-7258(96)00062-9. [ Links ]
8. Casella ML, Parody JP, Ceballos MP, et al. Quercetin prevents liver carcinogenesis by inducing cell cycle arrest, decreasing cell proliferation and enhancing apoptosis. Mol Nutr Food Res 2014;58:289-300. DOI: 10.1002/mnfr.201300362. [ Links ]
9. Espandiari P, Robertson LW, Srinivasan C, et al. Comparison of different initiation protocols in the resistant hepatocyte model. Toxicology 2005;206:373-81. DOI: 10.1016/j.tox.2004.07.014. [ Links ]
10. Álvarez ML, Cerliani JP, Monti J, et al. The in vivo apoptotic effect of interferon alfa-2b on rat preneoplasia liver involves bax protein. Hepatology 2002;35:824-33. DOI: 10.1053/jhep.2002.32099. [ Links ]
11. Solt D, Farber E. New principle for the analysis of chemical carcinogenesis. Nature 1976;263:701-3. DOI: 10.1038/263701a0. [ Links ]
12. Solt DB, Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol 1977;88:595-618. [ Links ]
13. Vera MC, Pisani GB, Biancardi ME, et al. Comparison of two chemical models to induce hepatic preneoplasia in male Wistar rats. Ann Hepatol 2015;14:259-66. [ Links ]
14. Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington, D.C.: National Academies Press; 2010. [ Links ]
15. Imai T, Masui T, Ichinose M, et al. Reduction of glutathione S-transferase P-form mRNA expression in remodeling nodules in rat liver revealed by in situ hybridization. Carcinogenesis 1997;18:545-51. DOI: 10.1093/carcin/18.3.545. [ Links ]
16. Greenwell A, Foley JF, Maronpot RR. An enhancement method for immunohistochemical staining of proliferating cell nuclear antigen in archival rodent tissues. Cancer Lett 1991;59:251-6. DOI: 10.1016/0304-3835(91)90149-C. [ Links ]
17. Damsch S, Eichenbaum G, Tonelli A, et al. Gavage-related reflux in rats: Identification, pathogenesis, and toxicological implications. Toxicol Pathol 2011;39:348-60. DOI: 10.1177/0192623310388431. [ Links ]
18. Mlyazaki M, Wahid S, Sato J. In vivo and in vitro test for growth potential of liver cells from rats during early stage of hepatocarcinogenesis by 3'-methyl-4- dimethylaminoazobenzene. J Cancer Res Clin Oncol 1989;115:1-8. DOI: 10.1007/BF00391592. [ Links ]
19. Moore MA, Hacker HJ, Bannasch P. Phenotypic instability in focal and nodular lesions induced in a short term system in the rata liver. Carcinogenesis 1983;4:595-603. DOI: 10.1093/carcin/4.5.595. [ Links ]
20. Junqueira LCU, Cossermelli WS, Brentani RR. Differential staining of collagen type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jap 1978;41:267-74. DOI: 10.1679/aohc1950.41.267. [ Links ]
21. Archana M, Yogesh TL, Kumaraswamy KL. Various methods available for detection of apoptotic cells - A review. Indian J Cancer 2013;50:274-83. DOI: 10.4103/0019-509X.118720. [ Links ]
22. Schulte-Hermann R, Bursch W, Kraupp-Grasl B, et al. Cell proliferation and apoptosis in normal liver and preneoplastic foci. Environ Health Perspec 1993;101:87-90. DOI: 10.1289/ehp.93101s587. [ Links ]
23. Singha O, Kengkoom K, Chaimongkolnukul K, et al. Pulmonary edema due to oral gavage in a toxicological study related to aquaporin-1, -4 and -5 expressions. J Toxicl Pathol 2013;26:283-91. DOI: 10.1293/tox.26.283. [ Links ]
24. Hadjiolov N, Bitsch A, Neumann HG. Early initiating and promoting effects in 2-AAF-induced rat liver carcinogenesis: An immunohistochemical study. Cancer Lett 1995;98:39-46. DOI: 10.1016/S0304-3835(06)80008-X. [ Links ]
25. Pogribny IP, Muskhelishvili L, Tryndyak VP, et al. The role of epigenetic events in genotoxic hepatocarcinogenesis induced by 2-acetylaminofluorene. Mutat Res 2011;722:106-13. DOI: 10.1016/j.mrgentox.2010.02.011. [ Links ]
26. Bannasch P, Klimek F, Mayer D. Early bioenergetic changes in hepatocarcinogenesis: Preneoplastic phenotypes mimic responses to insulin and thyroid hormone. J Bioenerg Biomembr 1997;29:303-13. DOI: 10.1023/A:1022438528634. [ Links ]
![]() Correspondence:
Correspondence:
Alejandra Beatriz Quintana.
Morphology Area and Physiology Department.
Facultad de Ciencias Bioquímicas y Farmacéuticas.
Universidad Nacional de Rosario.
Suipacha, 535/579. S2002LRK Rosario, Santa Fe. República Argentina
e-mail: aquintan@fbioyf.unr.edu.ar
Received: 04-01-2016
Accepted: 06-05-2016