Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.9 Madrid sep. 2016
https://dx.doi.org/10.17235/reed.2015.3912/2015
DOI: 10.17235/reed.2015.3912/2015
REVIEW
Endoscopic management of malignant biliary stenosis. Update and highlights for standard clinical practice
Tratamiento endoscópico de las estenosis malignas de la vía biliar. Puesta al día y puntos más relevantes para la práctica clínica habitual
María José Domper-Arnal and Miguel Ángel Simón-Marco
Department of Digestive Diseases. Hospital Clínico Universitario. Zaragoza, Spain
ABSTRACT
The present review describes the various indications of biliary stent placement in patients with biliary malignancies. It deals in depth with biliary accesses and their effectiveness, as well as with the use of different stents according to lesion type and expected patient survival. For liver hilum lesions, which are somewhat more complex, the usefulness of and need for unilateral or bilateral drainage is assessed, as it is the most appropriate method. All in all, this is an up-to-date literature review that may help clinicians in their daily decision-making, as well as to improve and optimize patient outcomes.
Key words: Biliary tract cancer. Endoscopic retrograde cholangiopancreatography. Stents. Biliary tract surgical procedures. Palliative therapy.
RESUMEN
En la presente revisión se describen las diferentes indicaciones para la colocación de prótesis biliares en pacientes con neoplasias malignas de la vía biliar. Se profundiza en los accesos a la vía biliar y en la efectividad de los mismos, así como en el uso de las diferentes prótesis en función del tipo de lesión y de la expectativa de sobrevida del paciente. En las lesiones de hilio hepático, algo más complejas, se valoran la utilidad y necesidad de un drenaje uni- o bilateral y el método más apropiado. En conjunto, se trata de una revisión actual de la literatura que puede ayudar al clínico a la toma de decisiones en su práctica diaria y a mejorar y optimizar los resultados en los pacientes.
Palabras clave: Cáncer de vías biliares. Colangiopancreatografía retrógrada endoscópica. Prótesis. Procedimientos quirúrgicos de la vía biliar. Tratamientos paliativos.
Introduction
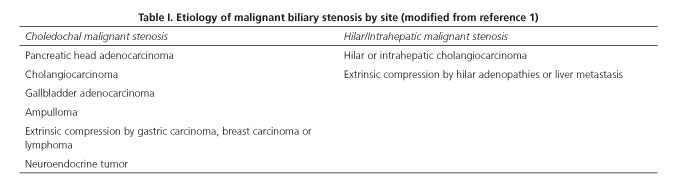
The etiology of malignant biliary stenosis varies according to localization. Among liver hilum malignancies primary cholangiocarcinoma is the most common one, whereas pancreatic adenocarcinoma predominates in the distal bile duct (1) (Table I). Jaundice reflects an advanced stage of disease and is a marker of poor prognosis with scarce curative potential. In this setting, the key goal for stent placement is palliative in nature, but the procedure is increasingly indicated as a preoperative measure for surgery with curative intent, or to achieve biliary patency in patients undergoing cancer therapy who will subsequently be reassessed and screened for surgery. Biliary stents play a key role for symptom relief and improved quality of life, but have failed to demonstrate higher survival rates (2).
Before the endoscopic procedure for biliary stent placement we must assess a number of factors besides the neoplasm itself: patient clinical status, life expectancy, and stent indication, also considering cost-effectiveness.
Material and Methods
Biliary drainage approach
Several strategies may be used to access the bile duct: endoscopic, percutaneous (interventionist radiology) or surgical. The endoscopic option is most useful and is considered as the primary option for biliary drainage (3).
The first description of biliary drainage for tumor-related obstructive jaundice using endoscopic retrograde cholangiopancreatography (ERCP) was performed by Soehendra in 1979. Spear and Cotton, in their papers published in The Lancet journal in 1987 and 1994, respectively, showed a greater effectiveness and lower complication rate for endoscopic versus percutaneous drainage. Compared to surgical drainage, the endoscopic approach could not maintain patency with equal efficacy, but complication rate was lower. No significant differences in mortality were seen between biliary approaches (3-6).
Biliary drainage using interventionist radiology is usually a second option after a previously failed endoscopic attempt in standard clinical practice, or a complementary (rendezvous) strategy. Speer et al. demonstrated a higher success rate for the endoscopic versus percutaneous approach (81% vs. 61%; p = 0.017), with lower mortality at 30 days of follow-up in the endoscopic drainage group (15% vs. 33%; p = 0.016). The mortality associated with the percutaneous procedure arose from puncture-related complications, including bleeding, liver laceration and bile leakage (3, 5).
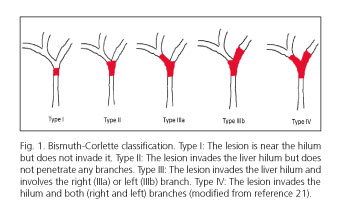
Regarding lesions at the liver hilum ERCP becomes more complex, as more than just one stent is often required in a difficult-to-access area with restricted maneuverability (3). Bismuth-Corlette's type-III (lesions reaching the confluence of hepatic ducts and a primary branch) and type-IV (lesions reaching the confluence of hepatic ducts and both primary branches) hepatic hilar neoplastic lesions (Fig. 1) are the most common ones, with rates of up to 60-70% or even higher, and most difficult to manage endoscopically. Some authors recommend the percutaneous approach for these lesions, particularly in less experienced centers or cases with challenging endoscopic access (7).
Of late, echoendoscopy-guided biliary drainage is being used as second option. While other approaches exist, biliary drainage is mainly performed transgastrically via the left intrahepatic bile duct. This is an extremely useful method for patients with anatomic changes, such as those having undergone gastrectomy or duodenopancreatectomy. Another form of echoendoscopy-guided drainage is rendezvous in combination with ERCP when access through the papilla of Vater has failed. These drain approaches are highly complex and should be attempted at third-level hospitals with highly trained endoscopists in ERCP and endoscopic ultrasounds (7-9).
Plastic versus metallic stents
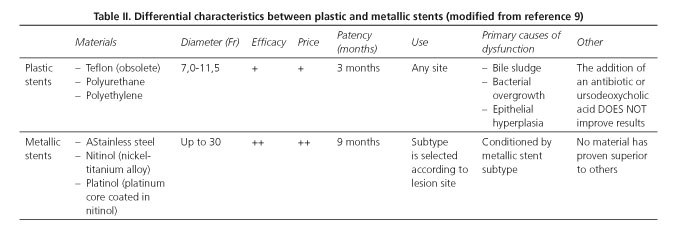
The material a biliary stent is made of conditions its applicability, effectiveness and potential limitations. The primary characteristics differentiating plastic from metallic stents are summarized in table II (10-12).
Different classic studies have shown that metallic stents are superior in terms of permeability (13), symptom-free survival, shorter hospital stay and lower cost (14), both for distal and hepatic hilar lesions (15,16).
A meta-analysis was recently performed comparing the use of plastic and metallic biliary stents in patients with irresectable malignant biliary obstruction (2). All these studies were controlled clinical trials. A total of 24 studies were included, 13 of them in patients with distal biliary malignancy, and 6 in patients with hepatic hilum neoplasms. Distal biliary malignancies included patients receiving both coated and partially coated metallic stents, but most (eight) studies involved uncoated stents. For hilar lesions all metallic stents were of the uncoated variety, and a high percentage of studies (5/6) included more patients with complex (Bismuth III or IV) lesions in the metallic versus plastic stent group. Final Results showed that, when compared to plastic stents, metallic stents are less prone to occlusion (RR: 0.27; 95% CI: 0.13-0.60), although no significant differences in mortality at 30 days were observed between both groups (RR: 0.74); 95% CI: 0.47-0.17) regarding all types of biliary stenosis. Overall, metallic stents are more effective and have fewer complications regardless of lesion type. In the subgroup with distal biliary malignant stenosis successful insertion (RR: 1.15; 95% CI: 0.56-2.37) and acute cholangitis (RR: 0.52; 95% CI: 0.25-1.09) rates were no different between both stents. Possibly, in this anatomic location the benefits of greater adaptability and flexibility provided by metallic stents are not so obvious, given the favorable anatomy of the distal bile duct (2).
From all the above we may conclude that metallic stents are the treatment of choice for malignant biliary obstruction. Their successful placement rate is similar to that of plastic stents but they also last longer, have fewer complications, reduce hospital admissions and mean stay, decrease the number of endoscopic procedures, and diminish overall costs (1, 2, 10, 17, 18).
Of all the variables discussed by prior studies only life expectancy was shown to be related to the use of metallic stents in terms of cost-effectiveness. Using a metallic stent for a patient with a life expectancy below 3 months was deemed as less cost-effective by virtually all studies. However, a recently reported clinical trial in 219 patients studied through 5 years in 18 Dutch and Belgian hospitals may change this approach. This prospective, randomized study included patients with irresectable malignant stenosis of the extra-hepatic bile duct who received a plastic, uncoated metallic or partly coated metallic stent for jaundice palliation. Follow-up lasted for about 1 year. Fifty-two percent of patients had metastatic disease at diagnosis, and mean survival was 109 days. Endoscopy materials, staff, and hospital stay costs were analyzed per patient. For patients with a life expectancy equal or inferior to 3 months no statistically significant differences were seen in cost-effectiveness between plastic and metallic stents (€6,555 vs. €5,719, p = 0.4), as was also the case for subjects with metastasis at diagnosis (€6,593 vs. €6,179, p = 0.69). The authors concluded that metallic stents should be routinely used for all patients (2, 10, 17-19).
Coated metallic versus uncoated metallic stents
Uncoated metallic stents adhere to bile duct walls and, since neoplasms naturally tend to grow into the stent through the mesh, bile drain becomes obstructed and stents cannot be withdrawn. This type of metallic stents is the only one to be used for hilar lesions. However, these stents represent just an option among the other types for lesions in the distal bile duct (1, 2, 11, 12, 17).
Coated metallic stents do not adhere to biliary walls, hence the virtual space extant between the external coat material and the bile duct wall may become a real space over time because of bile sludge deposition and an epithelial response to stent friction, mainly at stent ends. This allows potential stent withdrawal as well as stent migration after placement. They should not be used for hilar lesions since they may occlude collateral or intrahepatic branches, and trigger acute cholangitis. Furthermore, at the distal level cholecystitis and acute pancreatitis events have been described that may limit their use (1, 2, 17).
Amongst coated metallic stents we may distinguish fully coated stents, where the external coating includes the whole surface to both ends, and partly coated stents, where the mesh directly contacts the bile duct wall on their proximal and distal 5 mm-long ends. Partly coated stent design has been constantly revised to improve migration rates using "anti-migration" systems involving the gradual tapering of the uncoated ends and a reduction in axial force, which provides a lower tendency to migrate inside the bile duct (20).
Saleem et al. used a meta-analysis to compare the efficacy of coated versus uncoated metallic stents for the palliative management of malignant distal biliary stenosis. The meta-analysis included 5 randomized clinical trials (2 clinical trials used fully coated metallic stents and 3 used partly coated metallic stents) for a total of 781 patients. The most common etiology was pancreatic adenocarcinoma (69% of cases). Mean follow-up was 7 months, and 2 studies used the percutaneous approach for stent placement. Only 2 studies were included in the patient survival analysis. Patients receiving coated stents survived longer (51.18 days) than patients treated with uncoated stents (95% CI: 15.22-87.14; p = 0.01), a finding not shown by prior studies. Patency was superior for coated versus uncoated stents, with a mean difference of 60.56 days (95% CI: 25.96-95.17; p = 0.00). Coated stent dysfunction causes included choledochal epithelial reaction followed by cell overgrowth, bile sludge, and migration; uncoated stent dysfunction causes included tumor ingrowths through the mesh. In both cases findings were similar to those seen in other studies. Finally, the authors found no significant differences in acute cholecystitis (RR: 1.27; 95% CI: 0.41-3.92; p = 0.67) or acute pancreatitis (RR: 1.27; 95% CI: 0.25-6.39; p = 0.77) rates between both groups, which was seemingly inconsistent with the preconceived notion on this subject. Lastly, the sub-analysis comparing the use of fully versus partly coated stents found no statistically significant differences in any of the study endpoints (21).
Two years later, 2 meta-analyses were reported that compared coated versus uncoated metallic stents with similar Results (22, 23). Yang et al. performed the research to compare both stents using 11 studies (8 randomized controlled clinical trials, 3 prospective cohort studies) for a total of 684 patients with coated stents and 692 with uncoated stents for the treatment of malignant GI tract obstructions. No significant differences were found between coated and uncoated stents in terms of biliary permeability (HR 0.73; 95% CI: 0.41-1.32) or patient survival (HR 0.99; 95% CI: 0.77-1.28). This meta-analysis assessed exactly the same studies as the one by Saleem et al., but also included all the studies with available data regarding the analysis endpoints even if they did not involve all participants, and differences between coated and uncoated stents were not significant here (22).
In our setting a prospective, multicenter, non-randomized study was performed involving 32 Spanish hospitals to evaluate the efficacy and safety of partly coated metallic stents for the management of extrahepatic irresectable malignant biliary stenosis. Enrollment included 199 patients and mean follow-up was 18 months. The primary cause of biliary obstruction was pancreatic cancer (122 patients), and 84.6% of patients had in situ gallbladder. In all, 74% of stents remained patent at 6 months, and only one procedure was needed to relieve jaundice in most subjects. As regards Results, no patient had tumor growth through the mesh, stents migrated in 5% of patients (fewer than in prior studies: 4.9-13%), and only carrying a previous stent was related to higher migration rates in the multivariate analysis (OR 4.5; 95% CI: 1.1-17.8; p = 0.006). Epithelial overgrowth causing stent obstruction was seen in 5.5% of participants (much fewer than in prior studies). Acute cholecystitis developed in 2% of subjects, with other studies having reported proportions of up to 10%. Mortality was 2%, similar to other studies. This paper demystifies some complications that may raise doubts on the usefulness of metallic coated stents in clinical practice, and demonstrates their high effectiveness and safety (24).
Regarding the use of an anti-migration system in partly coated metallic stents, these stents were compared to uncoated ones fitted with the same anti-migration feature. In a prospective, randomized study of 120 patients with irresectable pancreatic adenocarcinoma in 22 third-level sites no differences were found in mean patient survival between both groups (mean: 285 and 223 days, respectively; p = 0.68). However, in the partially coated stent group stent patency was significantly higher (mean: 187 vs. 132 days, respectively; p = 0.043). In no patient was migration found to be the cause of stent dysfunction, regardless of stent type (20). Recent studies also advocate for this anti-migration system in order to minimize stent dysfunction rates secondary to metallic stent mobility (1,25).
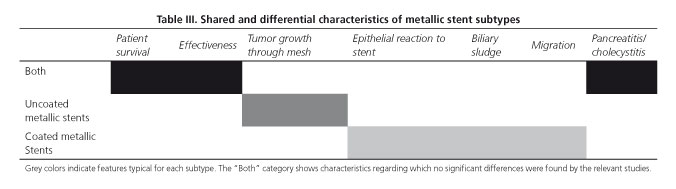
To sum it all up, table III lists the advantages and disadvantages of both coated and uncoated metallic stents.
Preoperative biliary drainage
Surgery is the treatment of choice for potentially curable malignant biliary lesions. Surgery usually involves a complex procedure with high morbidity and mortality. It has been suggested that hyperbilirubinemia in these patients may facilitate the development of sepsis, endotoxemia, and intravascular coagulation, which in turn would trigger hypercatabolism and malnutrition, thus increasing postoperative complication rates and casting a gloom over an already poor prognosis (3). The usefulness of biliary drainage prior to resection surgery has been hence suggested.
Preoperative drainage. Preliminary studies
Siddiqui et al., in a 5-year-long retrospective study, assessed 241 patients with resectable pancreatic cancer or scheduled for neo-adjuvant therapy, and showed that metallic stents were safe and effective for preoperative biliary drainage. Mean time between stent placement and surgery to relieve hyperbilirubinemia and provide patients with an optimal nutritional status was 4 weeks (3, 26). With a retrospective design and lacking a control group, Singal et al. demonstrated that placing a metallic stent added no technical difficulties during surgery, and was a safe and effective measure in the setting of resectable or potentially resectable neoplastic lesions following cancer therapy (3, 27). Other similarly designed studies have shown that using a metallic stent leads to lower re-obstruction rates as compared to plastic stents, both in hilar (0% vs. 41%, Grunhagen et al., 2013) and distal (0% vs. 39%, Decker et al., 2011) lesions (3, 28, 29).
Preoperative drainage. Randomized studies
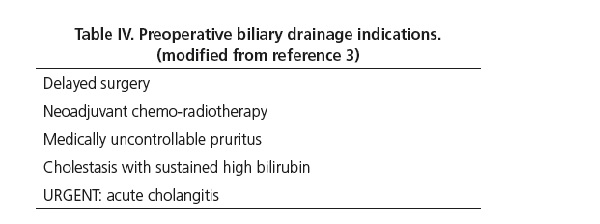
In 2010, a multicenter study in the Netherlands attempted to gain a deeper insight into this subject (30). This research team carried out a prospective study in patients with distal biliary obstruction of neoplastic origin, eligible for surgery (90% pancreatic head neoplasms), with total bilirubin below 15 mg/dL, and with a computed tomography (CT) scan revealing no metastasis or local invasion. Subjects were randomized into two groups: curative intent surgery within 7 days with no previous biliary drainage or ERCP for biliary drain placement using a plastic stent, followed by surgery after 4-6 weeks. The rate of severe complications requiring extended hospital stay or subsequent readmission, or leading to patient demise at 4 month's follow-up was 39% in the early surgery group and 74% in the endoscopic drain group (RR 0.54; 95% CI 0.41-0.71; p < 0.001 in the early surgery arm). No difference in mortality was found between groups. The main limitations of this study included a disproportionate rate of post-ERCP complications, mainly due to increased acute cholangitis (26%) among patients receiving preoperative drainage, and the fact that plastic rather than metallic stents were used (the superiority of self-expandable metallic stents in terms of effective drainage and lower complication rates is well known, and they add no difficulties to subsequent surgery) (17, 30). Other studies have reported that preoperative biliary drainage is more expensive (mean cost per patient of $15,616 vs. $11, 914) and provides a scarce benefit in quality-adjusted life years (QALYs) (0.337 vs. 0.343) (17). To conclude, curative intent surgery without prior biliary drainage is indicated for patients with bilirubin below 15 mg/dL and no acute cholangitis, provided the procedure may be accomplished within 7 days; otherwise, preoperative biliary drainage is indicated (3) (Table IV).
Neoadjuvant therapy and subsequent surgery
Pancreatic cancers are increasingly diagnosed as amenable to neoadjuvant therapy, with potential curative intent surgery being considered according to tumor response. In these patients bile duct patency achievement is indicated before cancer therapy since breakthrough infection or high bilirubin levels would lead to treatment discontinuation and delayed surgery. A metallic stent is recommended to avert early obstruction during the lapse of time between cancer therapy onset and re-staging for subsequent surgery, bearing in mind that neoadjuvant therapy has a mean duration of 104 days in reported studies. Since plastic stents have a mean service life of about 90 days, stent obstruction would be fairly common before surgery (17,31,32). In this context metallic stents are safe, effective, and cost-effective, and do not require their removal before surgery provided there is a free proximal choledochal segment to allow a surgical anastomosis (32).
Unilateral or bilateral drainage for malignant hilar lesions
Malignant hilar lesions have a serpiginous, motley morphology, and obstruction usually develops at various points in the biliary tree, which requires a distinct management (2).
Hilar lesions may be divided up into 4 types of increasing complexity (I-IV) according to the Bismuth-Corlette classification (33) (Fig. 1). Type-I lesions are usually solved with a unilateral drain, and type-IV lesions have a dismal prognosis, the predominantly obstructed lobe usually receiving a unilateral drain in clinical practice (2, 34, 35).
In order to compare unilateral versus bilateral drainage for non-resectable hilar lesions a meta-analysis was performed including 634 patients in 7 studies, which found no significant differences in mortality at 30 days (OR 0.73; 95% CI: 0.29-1.79) or stent obstruction rate regardless of stent type (metallic: OR: 1.09; 95% CI: 0.34-3.54; plastic: OR 1.86; 95% CI: 0.81-4.28), although studies were highly heterogeneous (I2 = 62%). Nor were statistically significant differences found between unilateral and bilateral drainage in terms of therapy failure (OR: 0.63; 95% CI: 0.31-1.28) or acute cholangitis rate (OR 0.61; 95% CI: 0.27-1.38) (2).
In other studies unilateral drainage is less effective and obtains lower success rates when compared to bilateral drainage (Liberato et al., success rate of 11.9% for unilateral versus 31.4% for bilateral drainage) (36). Much has been discussed on this topic, but the key lies in proper planning before ERCP execution using imaging techniques (CT or magnetic resonance imaging [MRI&]), with 3D images, to guide decisions and help come up with an "action plan" to facilitate subsequent therapy. Studies where bilateral drainage is more effective usually involve prior imaging (primarily CT or MRI scans) to guide the procedure, and patients selected using more objective criteria. More recent studies focus on whether one or two -even three- stents should be placed rather than on whether drainage should be unilateral or bilateral to drain over 50% of the hepatic volume. This is based on a study whose authors assessed which factors are predictive for drain effectiveness in patients with malignant hilar lesions (Bismuth II-IV). A total of 107 patients in 2 Parisian centers were retrospectively included. ERCP was guided by prior CT and/or MRI scans, which divided the liver in 3 main sectors (anterior right, posterior right, and left, according to the main bile duct branches running parallel to the portal vein), taking into account the total hepatic volume drained by each sector. In their Results, the authors observed that drain effectiveness, measured as a reduction in baseline bilirubin levels to at least half at 30 days during follow-up, was better when > 50% of hepatic volume was drained (OR: 4.5; 95% CI 1.07-6.46; p = 0.001), particularly for Bismuth III hilar stenosis, and that in patients reaching said drainage volume survival was higher (119 vs. 59 days, p = 0.005). Most often, draining > 50% of hepatic volume involved placing a drain in at least 2 of the 3 sectors. It was also shown that the draining of an atrophic lobe, with drainage for less than 30% of hepatic volume, represented no advantage and increased acute cholangitis rates following the procedure (OR 3.04; 95% CI: 1.24-7.48; p = 0.01) (2, 34).
From all this, in biliary drainage skipping atrophic sectors and using the number of stents necessary for draining at least 50% of total hepatic volume is most appropriate, particularly for Bismuth II and III lesions. Metallic stents should be used except for patients with survival below 3-6 months, and first-line access should be endoscopic, with the percutaneous option being considered for complex hilar lesions (Bismuth III-IV) (2, 10, 17, 34, 35).
Bilateral drainage primarily with metallic stents may be approached with two distinct techniques: stent-in-stent or side-by-side. Only a few studies assess which method is more effective or has fewer complications. Overall, metallic stent success rates using both Methods range from 73.3% to 100%, according to studies. Stent placement using the side-by-side technique is technically more complex and challenging, but stent patency rates over time are higher (35).
Conclusions
- The best way to gain access to the bile duct is the endoscopic approach for all cases except complex hilar lesions (usually Bismuth III-IV), where a percutaneous route may be considered as the first option in less experienced sites.
- Metallic stents are better than plastic stents for any biliary lesions and in all settings, their price being the limiting factor. They are considered to be not cost-effective in patients with estimated survival below 3 months, but recent papers raise doubts on this.
- The different metallic stent models available hardly differ in terms of patency duration and patient survival, their potential complications being their limiting factors. Uncoated stents should be used for the intrahepatic bile duct, whereas partly coated stents fitted with an anti-migration system are most promising in the extrahepatic bile duct.
- Preoperative biliary drainage should not be performed routinely and is to be avoided in the absence of an urgent indication when total bilirubin is lower than 15 mg/dL and elective surgery may be scheduled within 7 days. Subsequent surgery should not condition stent type. When needed, metallic stents should be used. For patients scheduled to receive neo-adjuvant therapy, a metallic stent should always be used to ensure biliary permeability.
- The endoscopic drainage of hilar lesions should be carried out according to Bismuth level and under the guidance of a prior imaging technique, preferably MRI. Number of stents and their location should be selected to provide a bile drainage > 50% of hepatic volume. Stents should not be placed in atrophic liver sectors. There is very little evidence regarding the best technique for bilateral drainage.
References
1. Blero D, Huberty, V, Devière, J. Novel biliary self-expanding metal stents: Indications and applications. Expert Rev Gastroenterol Hepatol 2015;9:359-67. DOI: 10.1586/17474124.2015.960395. [ Links ]
2. Sawas T, Al Halabi S, Parsi MA, et al. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: A meta-analysis. Gastrointest Endosc 2015;82:256-67.e7. DOI: 10.1016/j.gie.2015.03.1980. [ Links ]
3. Roque J, Ho SH, Goh KL. Preoperative drainage for malignant biliary strictures: Is it time for self-expanding meallis stents? Clin Endosc 2015;48:8-14. DOI: 10.5946/ce.2015.48.1.8. [ Links ]
4. Soehendra N, Reynders-Frederix V. Palliative biliary duct drainage. A new method for endoscopic Introduction of a new drain. Dtsch Med Wochenschr 1979;104:206-7. DOI: 10.1055/s-0028-1103870. [ Links ]
5. Speer AG, Cotton PB, Russell RC, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet 1987;2:57-62. [ Links ]
6. Smith AC, Dowsett JF, Russell RC, et al. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet 1994;344:1655-60. DOI: 10.1016/S0140-6736(94)90455-3. [ Links ]
7. Srinivasan I, Kahaleh M. Metal stents for hiliar lesions. Gastrointest Endosc Clin N Am 2012;22:555-65. DOI: 10.1016/j.giec.2012.05.009. [ Links ]
8. Kahaleh M, Artifon EL, Perez-Miranda M, et al. Endoscopic ultrasonography guided drainage: Summary of consortium meeting. May 21 2012, San Diego, California. World J Gastroenterol 2015;21:726-41. [ Links ]
9. Vila JJ, Pérez-Miranda M, Vázquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest Endosc 2012;76:1133-41. DOI: 10.1016/j.gie.2012.08.001. [ Links ]
10. Webb K, Saunders M. Endoscopic management of malignant bile duct strictures. Gastrointest Endosc Clin N Am 2013;23:313-31. DOI: 10.1016/j.giec.2012.12.009. [ Links ]
11. Dumonceau JM, Tringali A, Blero D, et al. Biliary stenting: Indications, choice of stents and Results. European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2012;44(3):277-98. DOI: 10.1055/s-0031-129163. [ Links ]
12. Dumonceau JM, Heresbach D, Devière J, et al. Biliary stents: Models and Methods for endoscopic stenting. Endoscopy 2011;43:617-26. DOI: 10.1055/s-0030-1256315. [ Links ]
13. Knyrim K, Wagner HJ, Pausch J, et al. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy 1993;25:207-12. DOI: 10.1055/s-2007-1010294. [ Links ]
14. Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage Methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc 1998;47:1-7. DOI: 10.1016/S0016-5107(98)70291-3. [ Links ]
15. Perdue DG, Freeman ML, DiSario JA, et al. Plastic versus self-expanding metallic stents for malignant hiliar biliary obstruction: A prospective multicenter observational cohort study. J Clin Gastroenterol 2008;42:1040-6. DOI: 10.1097/MCG.0b013e31815853e0. [ Links ]
16. Sangchan A, Kongkasame W, Pugkhem A, et al. Efficacy of metal and plastic stents in unresectable complex hiliar cholangiocarcinoma: A randomized controlled trial. Gastrointest Endosc 2012;76:93-9. DOI: 10.1016/j.gie.2012.02.048. [ Links ]
17. Rustagi T, Jamidar PA. Endoscopic treatment of manlignant strictures. Curr Gastroenterol Rep 2015;17:426. DOI: 10.1007/s11894-014-0426-9. [ Links ]
18. Itoi T, Sofuni A, Itokawa F, et al. Current status and issues regarding biliary stenting in unresectable biliary obstruction. Dig Endosc 2013;25(Suppl2):63-70. DOI: 10.1111/den.12062. [ Links ]
19. Walter D, Van Boeckel PG, Groenen MJ, et al. Cost efficacy of metal stents for palliation of extrahepatic bile duct obstruction in a randomized controlled trial. Gastroenterology 2015;149:130-8. DOI: 10.1053/j.gastro.2015.03.012. [ Links ]
20. Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: A randomized multicenter trial. Am J Gastroenterol 2013;108:1713-22. DOI: 10.1038/ajg.2013.305. [ Links ]
21. Saleem A, Leggett CL, Murad MH, et al. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc 2011;74:321-27. DOI: 10.1016/j.gie.2011.03.1249. [ Links ]
22. Yang Z, Wu Q, Wang F, et al. A systematic review and meta-analysis of randomized trials and prospective studies comparing covered and bare self-expandable metal stents for treatment of malignant obstruction in the digestive tract. Int J Med Sci 2013;10:825-35. DOI: 10.7150/ijms.5969. [ Links ]
23. Almadi MA, Barkun AN, Martel M. No benefit of covered vs. uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: A meta-analysis. Clin Gastroenterol Hepatol 2013;11:27-37. DOI: 10.1016/j.cgh.2012.10.019. [ Links ]
24. Gómez-Oliva C, Guarner-Argente C, Concepción M, et al. Partially covered self-expanding metal stent for unresectable malignant extrahepatic biliary obstruction: Results of a large prospective series. Surg Endosc 2012;26:222-9. DOI: 10.1007/s00464-011-1858-z. [ Links ]
25. Isayama H, Nakai Y, Kogure H, et al. Biliary self-expandable metallic stent for unresectable malignant distal biliary obstruction. Which is better: Covered or uncovered? Dig Endosc 2013;71-4. DOI: 10.1111/den.12078. [ Links ]
26. Siddiqui AA, Mehendiratta V, Loren D, et al. Self-expanding metal stents (SEMS) for preoperative biliary decompression in patients with resectable and borderline-resectable pancreatic cancer: Outcomes in 241 patients. Dig Dis Sci 2013;58:1744-50. DOI: 10.1007/s10620-012-2482-z. [ Links ]
27. Singal AK, Ross WA, Guturu P, et al. Self-expanding metal stents for biliary drainage in patients with resectable pancreatic cancer: Single center experience with 79 cases. Dig Dis Sci 2011;56:3678-84. DOI: 10.1007/s10620-011-1815-7. [ Links ]
28. Grunhagen DJ, Dunne DF, Sturgess RP, et al. Metal stents: A bridge to surgery in hilar cholangiocarcinoma. HPB (Oxford) 2013;15:372-78. DOI: 10.1111/j.1477-2574.2012.00588.x. [ Links ]
29. Decker C, Christein JD, Phadnis MA, et al. Biliary metal stents are superior to plastic stents for preoperative biliary decompression in pancreatic cancer. Surg Endosc 2011;25:2364-67. DOI: 10.1007/s00464-010-1552-6. [ Links ]
30. Van der Gaag NA, Rauws EA, Van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010;362:129-37. DOI: 10.1056/NEJMoa0903230. [ Links ]
31. Isayama H, Nakai Y, Kawakubo K, et al. Endoscopic retrograde cholangiopancreatography for distal malignant biliary stricture. Gastrointest Endosc Clin N Am 2012;22:479-90. DOI: 10.1016/j.giec.2012.04.024. [ Links ]
32. Aadam AA, Evans DB, Khan A, et al. Efficacy and safety of self-expandable metal stetns for biliary decompression in patient receiving neoadyuvant therapy for pancreatic cancer: A prospective study. Gastrointest Endosc 2012;76:67-75. DOI: 10.1016/j.gie.2012.02.041. [ Links ]
33. NCCN guidelines. www.nccn.or. [ Links ]
34. Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hiliar strictures: The role of liver volume assessment. Gastrointest Endosc 2010;72:728-35. DOI: 10.1016/j.gie.2010.06.040. [ Links ]
35. Lee TH, Moon JH, Park SH. Bilateral metallic stenting in malignant hiliar obstruction. Clin Endosc 2014;47:440-6. DOI: 10.5946/ce.2014.47.5.440. [ Links ]
36. Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: Efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol 2012;12:103. DOI: 10.1186/1471-230X-12-103. [ Links ]
![]() Correspondence:
Correspondence:
Ma José Domper-Arnal.
Department of Digestive Diseases.
Hospital Clínico Universitario.
Avda. San Juan Bosco, 15.
50009 Zaragoza, Spain
e-mail: mariajoseda_7@hotmail.com
Received: 07-07-2015
Accepted: 14-09-2015











 texto en
texto en