My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 n.3 Madrid Mar. 2017
https://dx.doi.org/10.17235/reed.2016.4338/2016
ORIGINAL PAPERS
Restoration of density of interstitial cells of Cajal in the jejunum of diabetic rats after quercetin supplementation
Flávia Cristina Vieira-Frez1, Juliana Vanessa Martins-Colombo-Perles3, David Robert-Linden2, Simon James Gibbons2, Heber Amilcar-Martins1, Débora Almeida-Brito-Romualdo3, Sara Raquel de-Souza3, Gleison Daion-Piovezana-Bossolani1 and Jacqueline Nelisis Zanoni3
1Department of Pharmacy. Universidade Estadual de Maringá. Avenida Colombo. Maringá, PR. Brazil.
2Department of Physiology and Biomedical Engineering and Enteric Neuroscience Program. Mayo Clinic College of Medicine. Rochester, MN. USA.
3Department of Morphological Sciences. Universidade Estadual de Maringá. Maringá, PR. Brazil
The project was funded by a grant from the Fundação Araucária (Support for Scientific and Technological Development in Paraná) and from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico - National Council for Scientific and Technological Development), Brazil.
ABSTRACT
Background: Interstitial cells of Cajal (ICC) are required for normal motility in the gastrointestinal tract. Depletion of ICC has been associated with diabetic gastroenteropathy.
Aim: To determine the effect of quercertin supplementation on anoctamin-1 (Ano1) immunoreactive ICC in the myenteric region (ICC-MY) and deep muscular plexus (ICC-DMP) in the jejunum of diabetic rats.
Methods: Thirty-two 90-day-old male Wistar rats were distributed into the following groups: normoglycemic (C), normoglycemic supplemented with quercetin (CQ; 40 mg daily), diabetic (D), and diabetic supplemented with quercetin (DQ; 40 mg daily). Diabetes was induced by streptozotocin injection. After 120 days, preparations of the jejunal muscular and submucosal layers were immunostained for Ano1 to visualize ICC. Evaluation of the immunofluorescence intensity as well as density of ICC was performed.
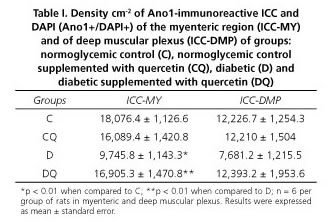
Results: The density of ICC-MY was 46% lower in group D compared to group C (p < 0.01); ICC-DMP were reduced by 37% (p > 0.05). After quercertin treatment, the densities of ICC-MY were significantly higher in the DQ group compared to group D (ICC-MY: 58%, p < 0.05). Supplementation with quercetin in normoglycemic animals (CQ) compared with group C did not significantly change the ICC density (p > 0.05).
Conclusions: In STZ-treated diabetic rats, diabetes promoted a reduction in the density of jejunal ICC-MY with no significant effect on ICC-DMP. Supplementation with quercetin (DQ) appeared to protect ICC-MY from depletion in diabetes possibly due to its antioxidant action.
Key words: Antioxidants. Diabetes mellitus. Interstitial cells of Cajal. Quercetin.
Introduction
Abnormalities in the motility of the gastrointestinal (GI) tract are a major complication of diabetes mellitus (DM) causing significant morbidity (1). Diabetes mellitus is commonly associated with gastrointestinal symptoms such as nausea, vomiting, diarrhea, bloating, epigastric burning sensation, abdominal discomfort and constipation (2,3). Diabetic gastroparesis is the best studied gastrointestinal complication of diabetes, since it affects many people with DM, causes significant morbidity and can interfere with glucose control (2,4). In animal models, DM is associated with enteric neuropathies (5-9) and loss of interstitial cells of Cajal (ICC) subpopulations in the GI tract (10-12).
ICC are non-neuronal cells in the GI muscularis propria, involved in generating the primary electrical pacemaker activity to control GI motility (13-15). These cells also form a bridge between enteric motor nerve terminals and smooth muscle cells (SMC). ICC also mediates enteric neural transmission due to the close association between ICC and enteric neurons and the presence of receptors for neurotransmitters transducing signals into post-junctional responses (16).
The present study investigated two separate functional groups of ICC in the GI tract: myenteric ICC (ICC-MY) and ICC of deep muscular plexus region (ICC-DMP). ICC-MY are located within the intermuscular space between the circular and longitudinal muscle layers. In the smooth muscles of the stomach and small intestine, ICC-MY act as pacemaker cells generating slow waves (17,18). ICC-DMP are present in the small intestine, situated in the deep muscular plexus in the circular muscle layers closest to the sub-mucosa (19). Unlike ICC-MY function, ICC-DMP mediate enteric neural transmission through synapse-like junctions from enteric nerve varicosities to SMC. These cells are densely innervated by enteric excitatory and inhibitory motor neurons which trigger stimuli in the deep musculature of the GI tract (19,20).
Oxidative stress plays a crucial role in the development of diabetic complications including neuropathies (21), and recent studies have demonstrated that oxidative stress plays a role in depletion and functional or structural disruption of ICC networks (22). ICC are traditionally identified by immunoreactivity for kit receptor tyrosine kinase (c-kit) (23), but recently a new marker for ICC, anoctamin-1 (Ano1, also known as TMEM16A or DOG1), has been identified as a key protein for electrical function of ICC (24,25). Ano1 is a calcium-activated chloride channel involved in the generation of the electrical slow wave, thereby displaying an important role in peristalsis of the GI tract (26). Changes in the expression of Ano1 protein in ICC may contribute directly to abnormal slow waves and gastrointestinal dysrhythmias (27,28). In the same way as kit, changes in expression of Ano1 reflect loss of ICC in gastrointestinal motility disorder (29).
Quercetin is a bioflavonoid antioxidant found in several fruits and vegetables. This polyphenolic flavonoid is a potent scavenger of reactive oxygen species (ROS) that exerts various pharmacological properties, including anti-cancer activity, anti-virus and anti-inflammatory effects, and reduces the risk of cardiovascular and renal diseases (30). Quercetin has been reported to protect diabetic neuropathy-induced damage via antioxidant and anti-apoptotic effects (31).
The present study examined the effect of quercetin supplementation on Ano1 expression and number of the ICC in the myenteric region and deep muscular plexus in the jejunum of diabetic rats.
Materials and methods
Animals
All of the experimental procedures were conducted in accordance to the ethical principles of the Brazilian Society of Science in Laboratory Animals (SBCAL) and approved by the Committee of Ethics in Animal Experimentation of the State University of Maringa - UEM (statement no. 053/2009). A group of 24 male 90-day-old Wistar rats (Rattus norvegicus) from the Central Animal Facility of UEM was used. The animals were distributed randomly into the following groups: normoglycemic control (C), normoglycemic control supplemented with quercetin (CQ), diabetic (D), and diabetic supplemented with quercetin (group DQ). They were kept in individual cages for a period of 120 days in a vivarium with a 12-h light/dark cycle and controlled temperature (24 ± 2 oC). They received food and water ad libitum and balanced standard Nuvital feed (Nuvilab, Colombo, PR, Brazil). For experimental supplementation of CQ and DQ groups, quercetin (Cromofarma, São Paulo, SP, and Brazil) was supplemented daily in the drinking water at a dose of 40 mg/day body weight. The non-supplemented animals (groups C and D) received water without quercetin, containing only NaOH 1M as the dilution vehicle for the flavonoid.
In order to calculate the dilutions required, ensuring that each animal in the CQ and DQ groups received an amount of 40 mg per day of quercetin in their drinking water, an average water intake for three consecutive days was determined. The animals were weighed weekly and mean of the group was used to maintain the amount of quercetin necessary to be solubilized in NaOH 1M with pH 7.4.
After a 14-hour fast, DM was induced in groups D and DQ by an intravenous injection of streptozotocin (35 mg/kg; Sigma, St. Louis, MO, USA) dissolved in citrate buffer, pH 4.5 (10 mm). After DM induction, glycemia was measured using the glucose oxidase method to confirm the establishment of the experimental model (32). Only animals with blood glucose levels above 250 mg/dL were selected for D and DQ groups.
Collection and processing of the material
After three months, animals were weighed and euthanized under thiopental anesthesia (40 mg/kg body weight intraperitoneally, Abbott Laboratories, Chicago, IL, USA). Blood was collected by cardiac puncture for the determination of glycemia levels. The animals received an intravenous penile injection of vincristine sulfate (0.5 mg/kg body weight; Tecnocris TM; Eurofarma/Zodiac Laboratories, São Paulo, SP, Brazil) two hours prior to euthanasia. The administration of this microtubule assembly inhibitor improves immunohistochemical labeling. Jejunal tissues were collected and processed for immunohistochemical analysis for Ano1.
Immunohistochemistry of ICC in whole-mount preparations
After laparotomy, jejuna from all animals were cut along the mesenteric border and rinsed in Phosphate Buffered Saline (PBS 0.1 M, pH 7.4). Briefly, tissue samples were fixed in cold acetone (1A1017.01.B; Prolab) for 15 min at 4 oC. After fixation, preparations were rinsed for 30 min in PBS that contained 10 mmol/L Na2HPO4, pH 7.4, and 150 mmol/L NaCl. Subsequently, the segments of jejunum were dissected under a Stemi DV4 stereomicroscope (Zeiss, Jena, Germany) to remove the mucosa tunica and submucosa layer and to acquire the whole-mount preparations for immunohistochemistry. In order to obtain the whole-mount preparations, the muscular layer was initially removed by dissection, and the mucosa was extracted by scraping with a small spatula. For immunostaining, non-specific binding was blocked by incubation in PBS (0.1 M, pH 7.4) that contained 10% normal donkey serum (UEM, Maringá, PR, Brazil) and 0.3% Triton X-100 (T8532, Sigma-Aldrich, Inc., St. Louis, MO, USA). The tissues sections were incubated for 48hours at 4oC with an anti-TMEM16A polyclonal antibody produced in rabbit (ab53212, 1:200 dilution; Abcam, Cambridge, MA, USA) diluted in PBS (0.1 M, pH 7.4) that contained 5% normal donkey serum and 0.3% Triton X-100. After washing with PBS (0.1 M, pH 7.4), specific labeling was detected by incubation with anti-rabbit IgG secondary antibody produced in goat (Alexa Fluor 488, A11008, 1:200 dilution; Invitrogen-Molecular Probes, Eugene, OR, USA) diluted in PBS (0.1 M, pH 7.4) that contained 2.5% normal donkey serum and 0.3% Triton X-100. The whole-mounts were then washed twice in PBS solution, mounted on slides with buffered glycerol (9:1), and stored in a refrigerator. Negative controls omitted the primary antibody.
Analysis of intensity of Ano1-immunoreactivity
The brightness intensity emitted by immunostaining for Ano1 was quantified using 30 random images of the jejunal whole mounts. The true color images were captured with a 1/600s exposure time through a 20x objective lens by a high resolution 5.0 Mega Pixel Moticam 2500 camera (Motic China Group Co., Shanghai, China), coupled to an Olympus BX40 optical fluorescence microscope (Olympus Co., Tokyo, Japan) and transferred to the PC using Motic Images Plus® 2.0ML software (Motic China Group Co.). ImageJ® version 1.43o image analysis software (National Institutes of Health, Bethesda, MD, USA) was used to quantify the brightness intensity emitted by immunostaining for Ano1 in stored images to determine the brightness (RGB). The analyses were evaluated blindly. All of the images were recorded before the evaluation, and the analysis was conducted by one researcher.
Immunohistochemistry of frozen histological sections
Approximately 3 cm of the jejunum was fixed in acetone (1A1017.01.B; Prolab) for 15 min at 4 oC. Then, samples were washed with PBS (0.1 M, pH 7.4) for five minutes. Afterward, tissues were cryoprotected in 18% sucrose (107651; Merck, Darmstadt, Germany) solution in PBS (0.1 M, pH 7.4) for 24 h. After cryoprotection, samples were embedded with O.C.T. 4583 compound (Tissue-Tek, Torrance, CA, USA), promptly frozen in liquid nitrogen and stored at -80 oC. Subsequently, semi-serial sections were made of 10 µm in a cryostat (Leica CM 1510, Germany), arranged on slides, and stored at -20 oC. For the tissue of each rat, a slide was made with seven sections to conduct immunofluorescence for Ano1, as described previously for whole-mount preparations. In contrast, the anti-rabbit IgG secondary antibody produced in goat (Alexa goat 568 A11008, 1:200 dilution; Invitrogen-Molecular Probes, Eugene, OR, USA) was used for the specific labeling. The tissues were mounted on slides using Prolong Gold antifade with 4',6-diamidine-2'-phenylindole dihydrochloride (DAPI) and buffered glycerol (9:1) (Molecular Probes) and stored in the refrigerator. The negative control was performed by omitting the primary antibody.
Cross-sectional image analysis
The images were captured using a high-resolution AxioCam camera (Zeiss, Jena, Germany) attached to a Plus Axioskop light microscope (Zeiss). The images were transferred to a computer using AxioVision 4 version 4.1 software. Image-Pro Plus version 4.5.0.29 image analysis software (Media Cybernetics, Silver Spring, MD, USA) was used for quantification. The immunohistochemical double labeling for Ano1 and DAPI were identified manually in 30 images captured with a 40x objective lens with five images from each of five 10 µm sections that were separated by at least 50 µm (34). For each animal, only Ano1-immunoreactive ICC were quantified, with the nucleus stained with DAPI, considered as Ano1+/DAPI+. The results are expressed as the number of ICC cm-2. All images were recorded before the evaluation, and the analysis was conducted by one researcher.
Statistical analysis
The results were analyzed using Statistic 7.1 and GraphPad Prism 3.1 and were expressed as mean ± standard error. The morphometric data were set out in blocks, followed by the Tukey test. For quantitative data, one-way analysis of variance (ANOVA) was performed, followed by Tukey test. The level of significance was 5%.
Results
Intensity of Ano1-immunoreactivity
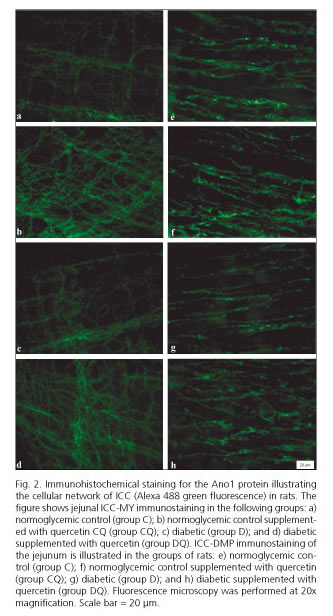
In the muscular layer of diabetic rats (group D), the intensity of Ano1-immunoreactivity in the myenteric region was 19.3% brighter compared to non-diabetic control rats (group C, p < 0.05) (Fig. 1). The intensity of Ano1-immunoreactivity was even brighter in the myenteric region of the DQ group compared to group D; it was specifically 37.9% higher (p < 0.05). For ICC in the deep muscular plexus region, the intensity of Ano1-immunoreactivity was 25.2% higher in the group CQ compared to the control group (group C, p < 0.05) (Fig. 1). The non-supplemented diabetic animals (group D, p < 0.05) displayed no differences in staining intensity compared to the control group (group C, p > 0.05) (Fig. 1). In the diabetic group supplemented with quercetin (group DQ) staining intensity was 25.7% higher than in the diabetic group (group D, p < 0.05) (Fig. 1). Representative photomicrographs for Ano1-immunoreactivity in the different groups are shown in figure 2.
Cross-sectional density of Ano1-immunoreactive ICC
^rND^1A01^nFlávia Cristina^sVieira-Frez^rND^1A03^nJuliana Vanessa^sMartins-Colombo-Perles^rND^1A02^nDavid^sRobert-Linden^rND^1A02^nSimon James^sGibbons^rND^1A01^nHeber^sAmilcar-Martins^rND^1A03^nDébora^sAlmeida-Brito-Romualdo^rND^1A03^nSara Raquel^sde-Souza^rND^1A01^nGleison^sDaion-Piovezana-Bossolani^rND^1A03^nJacqueline^sNelisis Zanoni^rND^1A01^nFlávia Cristina^sVieira-Frez^rND^1A03^nJuliana Vanessa^sMartins-Colombo-Perles^rND^1A02^nDavid^sRobert-Linden^rND^1A02^nSimon James^sGibbons^rND^1A01^nHeber^sAmilcar-Martins^rND^1A03^nDébora^sAlmeida-Brito-Romualdo^rND^1A03^nSara Raquel^sde-Souza^rND^1A01^nGleison^sDaion-Piovezana-Bossolani^rND^1A03^nJacqueline^sNelisis Zanoni^rND^1A01^nFlávia Cristina^sVieira-Frez^rND^1A03^nJuliana Vanessa^sMartins-Colombo-Perles^rND^1A02^nDavid^sRobert-Linden^rND^1A02^nSimon James^sGibbons^rND^1A01^nHeber^sAmilcar-Martins^rND^1A03^nDébora^sAlmeida-Brito-Romualdo^rND^1A03^nSara Raquel^sde-Souza^rND^1A01^nGleison^sDaion-Piovezana-Bossolani^rND^1A03^nJacqueline^sNelisis ZanoniORIGINAL PAPERS
Restoration of density of interstitial cells of Cajal in the jejunum of diabetic rats after quercetin supplementation
Flávia Cristina Vieira-Frez1, Juliana Vanessa Martins-Colombo-Perles3, David Robert-Linden2, Simon James Gibbons2, Heber Amilcar-Martins1, Débora Almeida-Brito-Romualdo3, Sara Raquel de-Souza3, Gleison Daion-Piovezana-Bossolani1 and Jacqueline Nelisis Zanoni3
1Department of Pharmacy. Universidade Estadual de Maringá. Avenida Colombo. Maringá, PR. Brazil.
2Department of Physiology and Biomedical Engineering and Enteric Neuroscience Program. Mayo Clinic College of Medicine. Rochester, MN. USA.
3Department of Morphological Sciences. Universidade Estadual de Maringá. Maringá, PR. Brazil
The project was funded by a grant from the Fundação Araucária (Support for Scientific and Technological Development in Paraná) and from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico - National Council for Scientific and Technological Development), Brazil.
ABSTRACT
Background: Interstitial cells of Cajal (ICC) are required for normal motility in the gastrointestinal tract. Depletion of ICC has been associated with diabetic gastroenteropathy.
Aim: To determine the effect of quercertin supplementation on anoctamin-1 (Ano1) immunoreactive ICC in the myenteric region (ICC-MY) and deep muscular plexus (ICC-DMP) in the jejunum of diabetic rats.
Methods: Thirty-two 90-day-old male Wistar rats were distributed into the following groups: normoglycemic (C), normoglycemic supplemented with quercetin (CQ; 40 mg daily), diabetic (D), and diabetic supplemented with quercetin (DQ; 40 mg daily). Diabetes was induced by streptozotocin injection. After 120 days, preparations of the jejunal muscular and submucosal layers were immunostained for Ano1 to visualize ICC. Evaluation of the immunofluorescence intensity as well as density of ICC was performed.
Results: The density of ICC-MY was 46% lower in group D compared to group C (p < 0.01); ICC-DMP were reduced by 37% (p > 0.05). After quercertin treatment, the densities of ICC-MY were significantly higher in the DQ group compared to group D (ICC-MY: 58%, p < 0.05). Supplementation with quercetin in normoglycemic animals (CQ) compared with group C did not significantly change the ICC density (p > 0.05).
Conclusions: In STZ-treated diabetic rats, diabetes promoted a reduction in the density of jejunal ICC-MY with no significant effect on ICC-DMP. Supplementation with quercetin (DQ) appeared to protect ICC-MY from depletion in diabetes possibly due to its antioxidant action.
Key words: Antioxidants. Diabetes mellitus. Interstitial cells of Cajal. Quercetin.
Introduction
Abnormalities in the motility of the gastrointestinal (GI) tract are a major complication of diabetes mellitus (DM) causing significant morbidity (1). Diabetes mellitus is commonly associated with gastrointestinal symptoms such as nausea, vomiting, diarrhea, bloating, epigastric burning sensation, abdominal discomfort and constipation (2,3). Diabetic gastroparesis is the best studied gastrointestinal complication of diabetes, since it affects many people with DM, causes significant morbidity and can interfere with glucose control (2,4). In animal models, DM is associated with enteric neuropathies (5-9) and loss of interstitial cells of Cajal (ICC) subpopulations in the GI tract (10-12).
ICC are non-neuronal cells in the GI muscularis propria, involved in generating the primary electrical pacemaker activity to control GI motility (13-15). These cells also form a bridge between enteric motor nerve terminals and smooth muscle cells (SMC). ICC also mediates enteric neural transmission due to the close association between ICC and enteric neurons and the presence of receptors for neurotransmitters transducing signals into post-junctional responses (16).
The present study investigated two separate functional groups of ICC in the GI tract: myenteric ICC (ICC-MY) and ICC of deep muscular plexus region (ICC-DMP). ICC-MY are located within the intermuscular space between the circular and longitudinal muscle layers. In the smooth muscles of the stomach and small intestine, ICC-MY act as pacemaker cells generating slow waves (17,18). ICC-DMP are present in the small intestine, situated in the deep muscular plexus in the circular muscle layers closest to the sub-mucosa (19). Unlike ICC-MY function, ICC-DMP mediate enteric neural transmission through synapse-like junctions from enteric nerve varicosities to SMC. These cells are densely innervated by enteric excitatory and inhibitory motor neurons which trigger stimuli in the deep musculature of the GI tract (19,20).
Oxidative stress plays a crucial role in the development of diabetic complications including neuropathies (21), and recent studies have demonstrated that oxidative stress plays a role in depletion and functional or structural disruption of ICC networks (22). ICC are traditionally identified by immunoreactivity for kit receptor tyrosine kinase (c-kit) (23), but recently a new marker for ICC, anoctamin-1 (Ano1, also known as TMEM16A or DOG1), has been identified as a key protein for electrical function of ICC (24,25). Ano1 is a calcium-activated chloride channel involved in the generation of the electrical slow wave, thereby displaying an important role in peristalsis of the GI tract (26). Changes in the expression of Ano1 protein in ICC may contribute directly to abnormal slow waves and gastrointestinal dysrhythmias (27,28). In the same way as kit, changes in expression of Ano1 reflect loss of ICC in gastrointestinal motility disorder (29).
Quercetin is a bioflavonoid antioxidant found in several fruits and vegetables. This polyphenolic flavonoid is a potent scavenger of reactive oxygen species (ROS) that exerts various pharmacological properties, including anti-cancer activity, anti-virus and anti-inflammatory effects, and reduces the risk of cardiovascular and renal diseases (30). Quercetin has been reported to protect diabetic neuropathy-induced damage via antioxidant and anti-apoptotic effects (31).
The present study examined the effect of quercetin supplementation on Ano1 expression and number of the ICC in the myenteric region and deep muscular plexus in the jejunum of diabetic rats.
Materials and methods
Animals
All of the experimental procedures were conducted in accordance to the ethical principles of the Brazilian Society of Science in Laboratory Animals (SBCAL) and approved by the Committee of Ethics in Animal Experimentation of the State University of Maringa - UEM (statement no. 053/2009). A group of 24 male 90-day-old Wistar rats (Rattus norvegicus) from the Central Animal Facility of UEM was used. The animals were distributed randomly into the following groups: normoglycemic control (C), normoglycemic control supplemented with quercetin (CQ), diabetic (D), and diabetic supplemented with quercetin (group DQ). They were kept in individual cages for a period of 120 days in a vivarium with a 12-h light/dark cycle and controlled temperature (24 ± 2 oC). They received food and water ad libitum and balanced standard Nuvital feed (Nuvilab, Colombo, PR, Brazil). For experimental supplementation of CQ and DQ groups, quercetin (Cromofarma, São Paulo, SP, and Brazil) was supplemented daily in the drinking water at a dose of 40 mg/day body weight. The non-supplemented animals (groups C and D) received water without quercetin, containing only NaOH 1M as the dilution vehicle for the flavonoid.
In order to calculate the dilutions required, ensuring that each animal in the CQ and DQ groups received an amount of 40 mg per day of quercetin in their drinking water, an average water intake for three consecutive days was determined. The animals were weighed weekly and mean of the group was used to maintain the amount of quercetin necessary to be solubilized in NaOH 1M with pH 7.4.
After a 14-hour fast, DM was induced in groups D and DQ by an intravenous injection of streptozotocin (35 mg/kg; Sigma, St. Louis, MO, USA) dissolved in citrate buffer, pH 4.5 (10 mm). After DM induction, glycemia was measured using the glucose oxidase method to confirm the establishment of the experimental model (32). Only animals with blood glucose levels above 250 mg/dL were selected for D and DQ groups.
Collection and processing of the material
After three months, animals were weighed and euthanized under thiopental anesthesia (40 mg/kg body weight intraperitoneally, Abbott Laboratories, Chicago, IL, USA). Blood was collected by cardiac puncture for the determination of glycemia levels. The animals received an intravenous penile injection of vincristine sulfate (0.5 mg/kg body weight; Tecnocris TM; Eurofarma/Zodiac Laboratories, São Paulo, SP, Brazil) two hours prior to euthanasia. The administration of this microtubule assembly inhibitor improves immunohistochemical labeling. Jejunal tissues were collected and processed for immunohistochemical analysis for Ano1.
Immunohistochemistry of ICC in whole-mount preparations
After laparotomy, jejuna from all animals were cut along the mesenteric border and rinsed in Phosphate Buffered Saline (PBS 0.1 M, pH 7.4). Briefly, tissue samples were fixed in cold acetone (1A1017.01.B; Prolab) for 15 min at 4 oC. After fixation, preparations were rinsed for 30 min in PBS that contained 10 mmol/L Na2HPO4, pH 7.4, and 150 mmol/L NaCl. Subsequently, the segments of jejunum were dissected under a Stemi DV4 stereomicroscope (Zeiss, Jena, Germany) to remove the mucosa tunica and submucosa layer and to acquire the whole-mount preparations for immunohistochemistry. In order to obtain the whole-mount preparations, the muscular layer was initially removed by dissection, and the mucosa was extracted by scraping with a small spatula. For immunostaining, non-specific binding was blocked by incubation in PBS (0.1 M, pH 7.4) that contained 10% normal donkey serum (UEM, Maringá, PR, Brazil) and 0.3% Triton X-100 (T8532, Sigma-Aldrich, Inc., St. Louis, MO, USA). The tissues sections were incubated for 48hours at 4oC with an anti-TMEM16A polyclonal antibody produced in rabbit (ab53212, 1:200 dilution; Abcam, Cambridge, MA, USA) diluted in PBS (0.1 M, pH 7.4) that contained 5% normal donkey serum and 0.3% Triton X-100. After washing with PBS (0.1 M, pH 7.4), specific labeling was detected by incubation with anti-rabbit IgG secondary antibody produced in goat (Alexa Fluor 488, A11008, 1:200 dilution; Invitrogen-Molecular Probes, Eugene, OR, USA) diluted in PBS (0.1 M, pH 7.4) that contained 2.5% normal donkey serum and 0.3% Triton X-100. The whole-mounts were then washed twice in PBS solution, mounted on slides with buffered glycerol (9:1), and stored in a refrigerator. Negative controls omitted the primary antibody.
Analysis of intensity of Ano1-immunoreactivity
The brightness intensity emitted by immunostaining for Ano1 was quantified using 30 random images of the jejunal whole mounts. The true color images were captured with a 1/600s exposure time through a 20x objective lens by a high resolution 5.0 Mega Pixel Moticam 2500 camera (Motic China Group Co., Shanghai, China), coupled to an Olympus BX40 optical fluorescence microscope (Olympus Co., Tokyo, Japan) and transferred to the PC using Motic Images Plus® 2.0ML software (Motic China Group Co.). ImageJ® version 1.43o image analysis software (National Institutes of Health, Bethesda, MD, USA) was used to quantify the brightness intensity emitted by immunostaining for Ano1 in stored images to determine the brightness (RGB). The analyses were evaluated blindly. All of the images were recorded before the evaluation, and the analysis was conducted by one researcher.
Immunohistochemistry of frozen histological sections
Approximately 3 cm of the jejunum was fixed in acetone (1A1017.01.B; Prolab) for 15 min at 4 oC. Then, samples were washed with PBS (0.1 M, pH 7.4) for five minutes. Afterward, tissues were cryoprotected in 18% sucrose (107651; Merck, Darmstadt, Germany) solution in PBS (0.1 M, pH 7.4) for 24 h. After cryoprotection, samples were embedded with O.C.T. 4583 compound (Tissue-Tek, Torrance, CA, USA), promptly frozen in liquid nitrogen and stored at -80 oC. Subsequently, semi-serial sections were made of 10 µm in a cryostat (Leica CM 1510, Germany), arranged on slides, and stored at -20 oC. For the tissue of each rat, a slide was made with seven sections to conduct immunofluorescence for Ano1, as described previously for whole-mount preparations. In contrast, the anti-rabbit IgG secondary antibody produced in goat (Alexa goat 568 A11008, 1:200 dilution; Invitrogen-Molecular Probes, Eugene, OR, USA) was used for the specific labeling. The tissues were mounted on slides using Prolong Gold antifade with 4',6-diamidine-2'-phenylindole dihydrochloride (DAPI) and buffered glycerol (9:1) (Molecular Probes) and stored in the refrigerator. The negative control was performed by omitting the primary antibody.
Cross-sectional image analysis
The images were captured using a high-resolution AxioCam camera (Zeiss, Jena, Germany) attached to a Plus Axioskop light microscope (Zeiss). The images were transferred to a computer using AxioVision 4 version 4.1 software. Image-Pro Plus version 4.5.0.29 image analysis software (Media Cybernetics, Silver Spring, MD, USA) was used for quantification. The immunohistochemical double labeling for Ano1 and DAPI were identified manually in 30 images captured with a 40x objective lens with five images from each of five 10 µm sections that were separated by at least 50 µm (34). For each animal, only Ano1-immunoreactive ICC were quantified, with the nucleus stained with DAPI, considered as Ano1+/DAPI+. The results are expressed as the number of ICC cm-2. All images were recorded before the evaluation, and the analysis was conducted by one researcher.
Statistical analysis
The results were analyzed using Statistic 7.1 and GraphPad Prism 3.1 and were expressed as mean ± standard error. The morphometric data were set out in blocks, followed by the Tukey test. For quantitative data, one-way analysis of variance (ANOVA) was performed, followed by Tukey test. The level of significance was 5%.
Results
Intensity of Ano1-immunoreactivity
In the muscular layer of diabetic rats (group D), the intensity of Ano1-immunoreactivity in the myenteric region was 19.3% brighter compared to non-diabetic control rats (group C, p < 0.05) (Fig. 1). The intensity of Ano1-immunoreactivity was even brighter in the myenteric region of the DQ group compared to group D; it was specifically 37.9% higher (p < 0.05). For ICC in the deep muscular plexus region, the intensity of Ano1-immunoreactivity was 25.2% higher in the group CQ compared to the control group (group C, p < 0.05) (Fig. 1). The non-supplemented diabetic animals (group D, p < 0.05) displayed no differences in staining intensity compared to the control group (group C, p > 0.05) (Fig. 1). In the diabetic group supplemented with quercetin (group DQ) staining intensity was 25.7% higher than in the diabetic group (group D, p < 0.05) (Fig. 1). Representative photomicrographs for Ano1-immunoreactivity in the different groups are shown in figure 2.
Cross-sectional density of Ano1-immunoreactive ICC
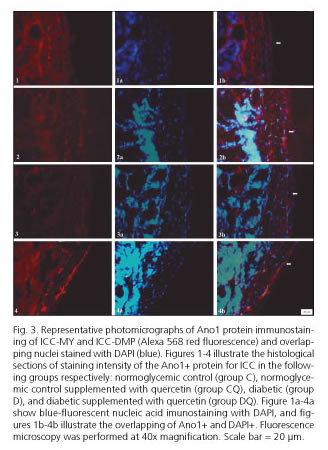
The density of ICC in the myenteric regions was lower in group D by 46% (p < 0.01) and 37% (p > 0.05) respectively, compared with group C (Table I). After quercetin treatment of diabetic rats, the density of ICC in the myenteric plexus was 58% (p < 0.01) and 38% higher (p > 0.05) in group DQ compared to group D, respectively (Table I). Supplementation with quercetin in normoglycemic animals (group CQ) compared to group C did not significantly affect the ICC density (p > 0.05) (Table I) in the myenteric or deep muscular plexus regions. Representative photomicrographs in the different groups are shown in figure 3.
Discussion
The experimental model of STZ-induced diabetes demonstrated a density reduction of ICC-MY and ICC-DMP, although only ICC-MY decreased significantly in diabetic rats (group D) in comparison to the normoglycemic rats (group C), as indicated by loss of Ano1-positive ICC. In this respect, we confirm that Ano1 can be used to report loss of ICC from the gastrointestinal tract under diabetic conditions. It was also observed that the remaining ICC-MY exhibited a greater intensity of Ano1-immunoreactivity under diabetic conditions.
Loss in ICC is an abnormality seen in diabetic gastroparesis (33) and several pathways may be involved, including: a) auto-oxidation of glucose; b) advanced glycation end-product (AGE) formation; c) polyol flux pathway; d) activation of protein kinase C (PKC) isoforms; and e) mitochondrial dysfunction (34). Increased oxidative stress derived from diabetes can lead to several complications due to dystrophy and degeneration of neurons, smooth muscle cells and ICC (33,34). The higher intensity of Ano1-immunoreactivity in ICC-MY of diabetic rats may indicate that when fewer ICC are present, Ano1 is upregulated to retain overall function with fewer cells in the presence of diabetes.
It was found that the intensity of Ano1-immunoreactivity and cell density of ICC-DMP between groups C and D were similar. ICC-DMP act as a messenger cell that mediates neuromuscular signaling between enteric neurons and smooth muscle, and have the capacity to reorganize their networks (19). Based on our study, ICC-MY in the rat jejunum are more susceptible to damage as a result of diabetes than ICC-DMP. This has not been shown for other studies on diabetic gastroenteropathy, since they are primarily focused on the stomach, where ICC-DMP are not present. However, it is known that ICC-DMP are somewhat preserved in c-kit and steel factor hypomorph mutant mice (e.g., W/Wv and Sl/Sld mice) (35,36); therefore, this population of cells may be less sensitive to effects of diabetes including loss of steel factor as presented by smooth muscle cells (37).
It was observed in the DQ group that ICC-MY Ano1-immunoreactivity was brighter when compared to group D. This observation is subject to the usual concerns with respect to using immune-labeling to report protein levels. However, the data that were scored blind are quite reproducible. Ideally, one would measure Ano1 protein by immunoblot, possibly normalized to kit to correct for the loss of ICC, but immunoblotting for rat Ano1 has not been shown, so this approach was not possible. The results were possibly due to a preservation of the density of ICC in DQ rats. Quercetin, a flavonoid, was used in this experiment due to its antioxidant property and because it is present in the components of the human diet (38). Generally, the antioxidant properties of quercetin are attributed to its ability to act as: a) suppressor of the formation of reactive oxygen species by inhibition of the enzyme systems responsible for the generation of free radicals (including cyclooxygenase, lipoxygenase and xanthine oxidase) (38,39); b) for binding of superoxide anions, singlet oxygen and hydroxyl radicals, and as a consequence reducing lipid peroxidation (40); and c) its function as a chelating agent for transition metals such as iron and copper (41), preventing the catalysis of free radical production (38,39). In addition, quercetin is also considered to be an aldose reductase enzyme inhibitor (42). All these associated factors could contribute to the preservation of ICC in diabetic conditions.
Diabetes induced an increase in the intensity of Ano1-immunoreactivity and a reduction in ICC-MY density. Supplementation with quercetin (DQ) exhibitedprotective effects on ICC numbers accompanied by an increase in Ano1-immunoreactivity possibly due to its antioxidant action.
References
1. Boland BS, Edelman SV, Wolosin JD. Gastrointestinal complications of diabetes. Endocrinol Metab Clin North Am 2013;42(4):809-32. [ Links ]
2. Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol 2011;9(1):5-12. DOI: 10.1016/j.cgh.2010.09.022. [ Links ]
3. Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: Current status and future directions. Neurogastroenterol Motil 2014;26:611-24. DOI: 10.1111/nmo.12330. [ Links ]
4. Horváth VJ, Izbéki F, Lengyel C, et al. Diabetic gastroparesis: Functional/morphologic background, diagnosis, and treatment options. Curr Diab Rep 2014;14:527. DOI: 10.1007/s11892-014-0527-8. [ Links ]
5. Farrugia G. Histologic changes in diabetic gastroparesis. Gastroenterol Clin North Am 2015;44(1):31-8. DOI: 10.1016/j.gtc.2014.11.004. [ Links ]
6. Ordög T, Hayashi Y, Gibbons SJ. Cellular pathogenesis of diabetic gastroenteropathy. Minerva Gastroenterol Dietol 2009;55(3):315-43. [ Links ]
7. Zanoni J, Miranda-Neto M, Bazotte, R. Morphological and quantitative analysis of the neurons of the myenteric plexus of the cecum of streptozotocin-induced diabetic rats. Arq neuropsiquiatr 1997;55:696-702. DOI: 10.1590/S0004-282X1997000500004. [ Links ]
8. Zanoni J, Buttow N, Bazotte R, et al. Evaluation of the population of NADPH-diaphorase-stained and myosin-V myenteric neurons in the ileum of chronically streptozotocin-diabetic rats treated with ascorbic acid. Auton Neurosci 2003;104:32-8. DOI: 10.1016/S1566-0702(02)00266-7. [ Links ]
9. De Freitas P, Natali M, Pereira R, et al. Myenteric neurons and intestinal mucosa of diabetic rats after ascorbic acid supplementation. World J Gastroenterol 2008;14:6518-24. DOI: 10.3748/wjg.14.6518. [ Links ]
10. Ordög T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 2000;49(10):1731-9. DOI: 10.2337/diabetes.49.10.1731. [ Links ]
11. Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 2008;135:2055-64. DOI: 10.1053/j.gastro.2008.09.003. [ Links ]
12. Lin L, Xu LM, Zhang W, et al. Roles of stem cell factor on the depletion of interstitial cells of Cajal in the colon of diabetic mice. J Physiol Gastrointest Liver Physiol 2010;298:241-7. DOI: 10.1152/ajpgi.90706.2008. [ Links ]
13. Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 2014;94:859-907. DOI: 10.1152/physrev.00037.2013. [ Links ]
14. Blair PJ, Rhee PL, Sanders KM, et al. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil 2014;20:294-317. DOI: 10.5056/jnm14060. [ Links ]
15. Al-Shboul O. The importance of interstitial cells of Cajal in the gastrointestinal tract. Saudi J Gastroenterol 2013;19:3-15. DOI: 10.4103/1319-3767.105909. [ Links ]
16. Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol 2006;576(Pt 3):675-82. DOI: 10.1113/jphysiol.2006.117390. [ Links ]
17. Sanders K. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterol 1996;111:492-515. DOI: 10.1053/gast.1996.v111.pm8690216. [ Links ]
18. Hirst DG, Edwards FR. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacological Sciences 2004;96:1-10. DOI: 10.1254/jphs.CRJ04002X. [ Links ]
19. Lino S, Kazuhide H. Interstitial cells of Cajal are involved in neurotransmission in the gastrointestinal tract. Acta Histochem Cytochem 2006;39:145-53. DOI: 10.1267/ahc.06023. [ Links ]
20. Zhou D, Komuro T. Interstitial cells associated with the deep muscular plexus of the guinea-pig small intestine, with special reference to the interstitial cells of Cajal. Cell Tiss Res 1992;268:205-16. DOI: 10.1007/BF00318788. [ Links ]
21. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058-70. DOI: 10.1161/CIRCRESAHA.110. 223545. [ Links ]
22. Kashyap P, Farrugia G. Oxidative stress: Key player in gastrointestinal complications of diabetes. Neurogastroenterol Motil 2011;23(2):111-4. DOI: 10.1111/j.1365-2982.2010.01659.x. [ Links ]
23. Maeda H, Yamagata A, Nishikawa S, et al. Requirement of c-kit for development of intestinal pacemaker system. Development 1992; 116(2):369-75. [ Links ]
24. West R, Corless C, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107-13. DOI: 10.1016/S0002-9440(10)63279-8. [ Links ]
25. Espinosa I, Lee CH, Kim MK, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol 2008;32:210-18. DOI: 10.1097/PAS.0b013e3181238cec. [ Links ]
26. Hwang SJ, Blair PJ, Britton FC, et al. Expression of anoctamin 1/TMEM 16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 2009;587:4887-904. DOI: 10.1113/jphysiol.2009.176198. [ Links ]
27. Caldeira A, Calmeiro E, Moreno C, et al. Diabetic gastroparesis II-Its interest. Med Int 2009;16:71-7. [ Links ]
28. Mazzone A, Bernard CE, Strege PR, et al. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem 2011;286:13393-403. DOI: 10.1074/jbc.M110.196089. [ Links ]
29. Kashyap P, Gómez-Pinilla PJ, Pozo MJ, et al. Immunoreactivity for Ano1 detects depletion of Kit-positive interstitial cells of Cajal in patients with slow transit constipation. Neurogastroenterol Motil 2011;23(8):760-5. DOI: 10.1111/j.1365-2982.2011.01729.x. [ Links ]
30. Lu Q, Ji XJ, Zhou YX, et al. Quercetin inhibits the mTORC1/p70S6K signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in diabetic nephropathy. Pharmacol Res 2015;99:237-47. DOI: 10.1016/j.phrs.2015.06.006. [ Links ]
31. Gomes IBS, Pereira TMC, Meyrelles SS, et al. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids in Health and Disease 2014;13:184. DOI: 10.1186/1476-511X-13-184. [ Links ]
32. Bergmeyer HU, Bernet E. Determination of glucose-oxidase and peroxidase. In: Methods of Enzymatic analysis, editor. 2nd ed. New York, NY: Verlag Chemie-Academic Press; 1974:6. [ Links ]
33. The NIDDK Gastroparesis Clinical Research Consortium (GpCRC). Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterol 2011;140:1575-85. [ Links ]
34. Folli F, Corradi D, Fanti P, et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro-and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev 2011;7:313-24. DOI: 10.2174/157339911797415585. [ Links ]
35. Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 1994;1;480(Pt 1):91-7. DOI: 10.1113/jphysiol.1994.sp020343. [ Links ]
36. Ward SM, Burns AJ, Torihashi S, et al. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol 1995;269(6 Pt 1):C1577-85. [ Links ]
37. Horváth VJ, Vittal H, Lörincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of Cajal in murine diabetic gastroparesis. Gastroenterol 2006;130(3):759-70. DOI: 10.1053/j.gastro.2005.12.027. [ Links ]
38. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: An overview. Scient W J 2013;2013:162750. DOI: 10.1155/2013/162750. [ Links ]
39. Adewole SO, Caxton-Martins EA, Ojewole JA. Protective effect of quercetin on the morphology of pancreatic β-cells of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med 2006;4:64-74. [ Links ]
40. Nabavi SF, Russo GL, Daglia M, et al. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem 2015;179:305-10. DOI: 10.1016/j.foodchem.2015.02.006. [ Links ]
41. El Hajji H, Nkhili E, Tomao V, et al. Interactions of quercetin with iron and copper ions: Complexation and autoxidation. Free Radic Res 2006;40:303-20. DOI: 10.1080/10715760500484351. [ Links ]
42. Milackova I, Prnova MS, Majekova M, et al. 2-chloro-1,4-naphthoquinone derivative of quercetin as an inhibitor of aldose reductase and anti-inflammatory agent. J Enzyme Inhib Med Chem 2015;30:107-13. DOI: 10.3109/14756366.2014.892935. [ Links ]
![]() Correspondence:
Correspondence:
Jacqueline Nelisis Zanoni.
Department of Morphological Sciences.
Universidade Estadual de Maringá.
Av. Colombo, 5790 bloco O-33.
87020-900 Maringá, Brazil
e-mail: jnzanoni@uem.br
Received: 28-03-2016
Accepted: 29-10-2016