My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 n.5 Madrid May. 2017
https://dx.doi.org/10.17235/reed.2017.4630/2016
REVIEW
Intraductal papillary mucinous neoplasms and mucinous cystadenomas: current status and recommendations
Tumores papilares mucinosos intraductales y cistoadenomas mucinosos: postura actual y recomendaciones
Maria Moris and Michael B. Wallace
Department of Gastroenterology. Mayo Clinic. Jacksonville, Florida. USA
Disclosures: MM has nothing to disclose. MBW receives research funding from Olympus, Boston Scientific, and Ninepoint Medical. MBW received consulting fees in 2015 from Olympus and iLumen unrelated to this study.
ABSTRACT
The real prevalence of pancreatic cystic lesions remains unknown. The malignant potential of some of these lesions remains a cause for significant concern. Thus, it is mandatory to develop a strategy to clearly discriminate those cysts with a potential for malignant transformation from those that do not carry any significant risk. Intraductal papillary mucinous neoplasms and mucinous cystadenomas are mucinous cystic neoplasms with a known malignant potential that have gained greater recognition in recent years. However, despite the numerous studies that have been carried out, their differential diagnosis among other cysts subtypes and their therapeutic approach continue to be a challenge for clinicians. This review contains a critical approach of the current recommendations and management strategies regarding intraductal papillary mucinous neoplasms and mucinous cystadenomas, as well as highlighting the limitations exposed in current guidelines.
Key words: IPMNs. MCAs. Pancreatic cyst. Malignancy.
RESUMEN
La prevalencia real de las lesiones quísticas de páncreas sigue siendo una incógnita. El potencial de malignidad de algunas de estas lesiones supone una causa de preocupación significativa en la práctica clínica diaria. Por lo tanto, es necesario determinar una estrategia para poder discriminar claramente los quistes potencialmente malignos de aquellos que no suponen ningún tipo de riesgo. Los tumores papilares mucinosos intraductales y los cistoadenomas mucinosos son neoplasias quísticas mucinosas potencialmente malignas que han ido ganando mayor importancia y reconocimiento en los últimos años. Sin embargo, pese a los múltiples estudios que se han realizado hasta la fecha, su diagnóstico diferencial respecto a otros subtipos de quistes, así como su manejo terapéutico, continúan suponiendo un reto. Este manuscrito contiene una revisión crítica de las recomendaciones actuales y de las estrategias en el manejo de los tumores papilares mucinosos intraductales y los cistoadenomas mucinosos, así como hace hincapié en las limitaciones de las guías actuales.
Palabras clave: TPMI. CAM. Quistes pancreáticos. Malignidad.
Introduction
The term "pancreatic cystic lesion" (PCL) involves a heterogeneous group of pancreatic cysts including totally benign lesions such as pseudocysts and potentially malignant entities such as mucinous cystadenomas (MCAs) or intraductal papillary mucinous neoplasms (IPMNs). Currently, the real prevalence of PCLs remains unknown. Several studies have attempted to elucidate this matter, resulting in a wide spectrum of prevalence estimates ranging from 0.2% to 44.7% (1-4). This wide range is a consequence of significant heterogeneity with regard to the selected population, the type of imaging technique or the subtype of cysts described.
Despite this, there is general agreement regarding an increased trend of incidental PCLs diagnosed in recent years (5,6), mainly due to an aging population and the widespread use of high-resolution imaging technologies (7,8). The malignant potential of some, albeit a small proportion of these lesions, remains a cause for significant concern. Currently, the only accepted treatment is surgical resection, which is an aggressive approach, moreover considering that it may involve benign lesions (9). Thus, it is mandatory to develop a strategy to clearly discriminate those cysts with a potential for malignant transformation from those that do not carry any significant risk.
PCLs include a broad amalgam of cystic lesions including the so-called pancreatic cystic neoplasms (PCNs). There are four main types of PCNs: IPMNs, MCAs, serous cystadenomas and solid pseudopapillary neoplasms (Table I). As its name indicates, these lesions have a potential for malignant transformation (10) that broadly ranges from 1% to 36% (11-14).
Herein, we will focus on the most common mucinous cysts: the IPMNs and the MCAs. Due to their greater recognition (5) and the improved knowledge of their pathway to malignant transformation, these entities have gained increased attention in recent years. However, despite the numerous studies that have been carried out (15), their differential diagnosis among other PCL subtypes and the therapeutic approach continue to be a challenge for clinicians.
Intraductal Papillary Mucinous Neoplasms (IPMNs) and Mucinous Cystadenomas (MCAs)
IPMNs
IPMNs were reported for the first time in 1980 (16) and considered as an independent entity since 1996 (17). IPMNs are intraductal lesions characterized by a columnar mucin-secreting epithelium that may characteristically present with papillary projections into the pancreatic duct lumen (18). They encompass a spectrum of cystic neoplasms with diverse potential for malignancy, following a progressive pathway of low-grade dysplasia (LGD) to high-grade dysplasia (HGD) to invasive pancreatic ductal adenocarcinoma (19).
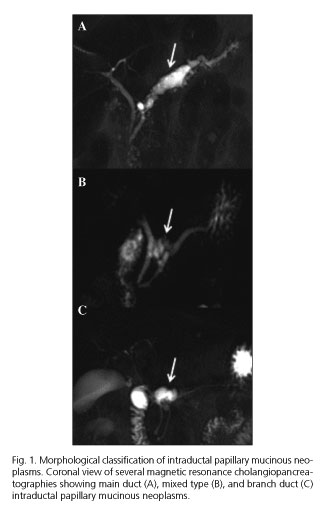
IPMNs can derive from the epithelium of the main pancreatic duct (MD) or the side branches (BD) (Fig. 1). This distinction has clinical relevance because MD involvement has been identified as a predictor of malignant transformation (20-23). A MD dilation over 9 mm is considered to be a "high-risk stigmata" whereas a dilation comprised between 5-9 mm is considered as a "worrisome feature" for malignancy (24). There is a third classification named "mixed type IPMN" that comprises both MD and BD involvement. It has been postulated that this category should not be considered as an independent type due to their same clinicopathologic behavior as MD-IPMNs. However, in recent consensus guidelines it remained a distinct subtype due to its interest from a pathological point of view (24,25). BD-IPMNs, on the other hand, are the main subject of debate nowadays. Initially, the main recommendation was to resect them due to their malignant potential. However, several recent studies have reported significantly lower rates of malignant transformation (ranging from 2-6%) (26-29), which has leaned the balance towards a more conservative approach.
IPMNs are also classified according to the histological subtype, which is associated with anatomic (MD versus BD) location. This classification has clinical importance because it predicts the IPMNs biological behavior (30). Four different subtypes have been described: gastric, intestinal, pancreatobiliary and oncocytic (24). The majority of MD-IPMNs are the intestinal subtype, which tends to progress to colloid carcinoma and has a better prognosis than conventional adenocarcinoma. On the other hand, BD-IPMNs are usually a gastric subtype that is mainly benign. However, if malignancy develops from this cell lining, it is a tubular carcinoma, which has a poor prognosis similar to pancreatic ductal adenocarcinoma (31).
IPMNs are usually diagnosed incidentally in middle aged and older patients (32) with a slightly higher prevalence in males (60%) (33). They are frequently located in the head of the pancreas but may also have a multifocal presentation throughout the gland (34). In MD-IPMNs the "fish mouth" sign, consisting of mucus extruding through the papilla, is a pathognomonic sign reported in 22 to 55% (35) of the cases. Moreover, the presence of mucus drainage through a pancreaticoduodenal fistula is seen in up to 2% of the cases and is suggestive of underlying malignancy (36).
The biomarkers seen in IPMNs include p16/CDKN2A, SMAD4 and TP53 (37,38). Moreover, the loss of expression of p16/CDKN2, CDKN1C and ppENK may also be observed in these lesions (39). Recently, several studies (40-42) have demonstrated the presence of GNAS (mainly in the intestinal subtype) and/or KRAS (pancreatobiliary subtype) mutations in up to 96% of IPMNs. GNAS mutations have become a promising diagnostic tool, as they seem to be selectively present in IPMNs and, consequently, absent in MCAs. Unfortunately, this GNAS mutation is not seen in every IPMN and, thereby, is still far from being considered as the gold standard for IPMN diagnosis.
Overall, one of the main concerns regarding IPMNs is their uncertain natural history. Initially, the majority of the publications reported surgically resected cohorts leading to an overestimation of their malignant potential secondary to selection bias. Recent studies, based on observational cohorts, have shown noticeable lower risks of malignancy (43). Thus, newer recommendations tend to follow a more conservative approach especially for the BD-IPMNs with no "worrisome features" (44).
MCAs
MCAs are a different type of PCNs that comprises 25% (45) of the resected lesions. The presence of an ovarian type stroma that supports a mucin-secreting columnar epithelium is a pathognomonic sign of this entity.
Although quite low, MCAs have a potential for malignant transformation as well. The inner epithelium of MCAs may present areas with pseudopyloric, gastric, foveolar and small and colonic intestinal differentiation. Taking into consideration the highest grade of cytological atypia, MCAs can be further categorized into LGD, HGD and invasive carcinoma, the latter resembling conventional pancreatic ductal adenocarcinoma (46). If invasive malignant disease arises in these lesions, the prognosis is poor with a reported post-surgical 5-year overall survival as low as 17% (47).
MCAs are predominantly diagnosed in asymptomatic female patients in their 4th-5th decade of life. Macroscopically, they are solitary homogeneous macrocystic lesions presenting in the distal (body and tail) gland that characteristically do not communicate with the pancreatic duct (48). Typically, they have septa and, occasionally, they may also present with calcifications or nodules (13), the latter suggesting malignancy (Fig. 2).
Due to their usual location in the distal (tail) gland (which generally allows a distal pancreatectomy with less secondary effects and comorbidities [49]), the typical presentation in young patients, and their poor prognosis if malignancy develops (47), surgical resection is primarily indicated (24). Despite this, a recent retrospective study evaluated 90 patients with a surgically resected MCA (50). Of these, only 6% were HGD and 4% were harbored invasive carcinoma, concluding that, in the absence of symptoms, MCAs smaller than 3 cm with no mural nodules or elevated tumor markers may not need resection. However, the authors suggested that validation with a prospective study is needed. Even if these results are validated, it seems that a life-long surveillance program for these, generally young patients, may carry significant costs, as well as anxiety for the patient, compared to surgical resection that does not need further follow-up based on current literature findings (51).
Several epithelial markers have been proposed for MCAs, such as carcinoembriogenic antigen (CEA) and cytokeratins 7, 8, 18 and 19, as well as the gastric foveolar type markers such as MUC5AC (with MUC1 being present in malignant MCAs). Also, K-ras, p53, RNF43 and SMAD4 mutations may be seen in dysplastic and invasive MCAs (40,52).
MCAs usually have an elevated CEA (53) in cyst fluid that can help to discriminate these lesions from serous cystadenomas (which also tend to present as solitary lesions but have a minimal risk of developing malignancy). However, IPMNs typically have high cyst CEA levels as well. Therefore, it may be difficult to differentiate between these two entities preoperatively (54). Another added challenge is the pancreatic duct communication that theoretically is only patent in IPMNs. Despite this, a study showed that at least 15% of pathologically confirmed MCAs had a communication between the lesion and the MD (13).
Whereas current guidelines recommend resection for all suspected MCAs, clinically suspected IPMNs must undergo further classification to discriminate which lesions should be resected (due to their high risk of developing malignancy) and which should undergo periodic surveillance. Hence, it is mandatory to design a preoperative strategy to categorize and discriminate among IPMNs and MCAs due to their different management and prognosis.
Diagnosis
Presently, the reported agreement between preoperative diagnosis and the pathology for PCLs is comprised between 68-78% (55-57). A combined strategy between the different imaging modalities as well as techniques that allow tissue/fluid sampling, such as endoscopic ultrasound (EUS), is currently recommended, at least for larger or worrisome lesions, to characterize and define these lesions (57,58).
Imaging studies
To date, there have been several studies that have reported the insufficient accuracy of current imaging modalities to discriminate PCLs (59,60). Despite this, magnetic resonance cholangiopancreatography (MRCP) is currently considered to be the best imaging technique for detection and characterization of both IPMNs and MCAs. This modality groups the routine contrast-enhanced magnetic resonance imaging with fast spin-echo sequences, allowing 3D reconstructions of the pancreatic and biliary trees. Overall, this technology has the ability to detect ductal communications as well as the presence of clinically relevant intracystic features such as mural nodules (61).
Recently, a study (62) showed no differences between MRCP and multidetector computed tomography for the characterization of these lesions. The combination of both modalities was not significantly better than each of them alone, however, the authors suggested that both techniques could be used together in selected difficult cases where no clear radiologic pattern is seen.
Even if MRCP and the multidetector computed tomography have the same accuracy, the absence of radiation in the case of MRCP offers more advantages especially in younger patients. Also, MRCP seems to carry lower risk of undesirable effects secondarily to contrast administration (63).
Endoscopic modalities
Recently, the ASGE published new guidelines regarding the role of endoscopy in the diagnosis of PCNs (64). These guidelines aimed to describe the utility of the different endoscopic modalities especially in those cases were no characteristic radiological features of the cysts are seen, and inconclusive information is obtained from the primary examinations.
Endoscopic retrograde cholangiography (ERCP) allows pancreatography, which may be helpful in the differential diagnosis among IPMNs and MCAs if communications or segmental strictures in the pancreatic duct are seen. However, these findings are not specific for PCNs as they can also be described in pseudocysts or chronic pancreatitis, respectively. One advantage of this technique is that tissue sampling can be performed via brush cytology or random biopsies. Nevertheless, a recent systematic review (65) that analyzed 483 IPMN patients demonstrated that ERCP-based cytology had a good specificity (97%) but an insufficient sensitivity (35%) with an overall accuracy of 93%. In this same review, lavage cytology was the best approach with a sensitivity of 46% and a specificity of 98%. New technologies for tissue sampling such as intrapancreatic videoscope with fiberoptic probes have been tested (66), showing excellent results with 100% sensitivity and specificity for detecting malignancy via irrigation cytology in patients with dilated MDs (67). Despite these promising results, ERCP is rarely indicated for PCN diagnosis due to the inherent risks related to the procedure and the current insufficient diagnostic accuracy.
EUS is currently the preferred endoscopic modality to diagnose, sample and follow-up PCNs. One of the main limitations, however, is the significant variability between observers (68) in the description of morphological findings. This can be implemented with the performance of fine needle aspiration (FNA) targeting cyst fluid, intracystic components, pancreatic ducts or lymph nodes (69,70). Moreover, it has been reported that the combined use of EUS-FNA with a high-resolution imaging modality can increase the diagnostic accuracy up to 54% (71). On the other hand, differentiation between intracystic mucus versus mural nodules is usually challenging in EUS. To address this matter, a recent study (72) reported that mural nodules tend to appear with ill-defined borders and a hyperechoic center, as opposed to mucus that usually presents with a hyperechoic rim and hypoechoic center. Recently, contrast-enhanced EUS has seemed to provide valuable information regarding the vascularity perfusion characteristically seen in mural nodules (73), contributing to the current problematic regarding the differential diagnosis with intracystic mucus. Lastly, several studies have demonstrated an increased efficacy of EUS if combined with through-the-needle confocal laser-induced endomicroscopy. In fact, a recent study (74) showed a diagnostic accuracy of 93% when discriminating mucinous cysts if cystoscopy was also added to this technique. Moreover, the results showed a superior diagnostic accuracy if both techniques were performed combined versus independently (87% laser-induced endomicroscopy and 83% cystcoscopy, respectively).
Cyst fluid
Cytology of cyst fluid is usually challenging due to the small fluid specimen in most cysts less than 1-2 cm diameter, insufficient presence of cells and cellular contamination secondary to the path of the needle (such as gastric or duodenal wall cells). Thereby, the sensitivity of cytology in cyst fluid has conventionally been very low. A recent meta-analysis (75) that reviewed 1,438 patients with a PCL reported an insufficient sensitivity of 54% and an optimal specificity of 93% to distinguish mucinous from non-mucinous cysts. To try to overcome the cytology deficiencies, one previously reported strategy was to redefine the concept of a "positive" sample as the presence of sufficient high-grade atypical cells that would not be quantitatively or qualitatively enough for cancer diagnosis (76). With this modified concept, the sensitivity and specificity to predict HGD or invasive carcinoma in mucinous cysts were 72% and 85% respectively. Another group (77) published similar results when an exclusive-IPMN cohort was analyzed with a sensitivity and specificity of 77% and 80%, respectively. Nevertheless, their sensitivity dropped to 67% if only BD-IPMNs of less than 3 cm were included in the sub-analysis.
Secondary to the insufficient accuracy of cytology in cyst fluid, several fluid markers have been tested and continue to be evaluated. The most common one is the CEA. There is general agreement that the usefulness of CEA solely lies in discriminating among mucinous and non-mucinous cysts (78); CEA levels > 800 ng/ml have been reported to carry a positive predictive value up to 94% and an overall diagnosis accuracy of 79% (79). Despite this, there is not a clear consensus regarding the optimal CEA cut-off value (80), with 192 ng/ml being the most commonly used (54) (sensitivity of 75%, specificity of 84%). Also, a recent study (81) evaluated the clinical significance of serial CEA measurements by analyzing 400 patients with PCNs who underwent several EUS-FNAs. In 17 (20%) patients, CEA fluctuations were noticed but these were not accompanied by significant EUS variability. Therefore, CEA level fluctuations do not seem to correlate with clinically significant changes.
Finally, molecular DNA analysis of cyst fluid is currently being developed. Several studies, including the PANDA study involving multiple centers, evaluated the presence of KRAS mutations or allelic imbalance to discriminate among mucinous and non-mucinous cysts, with poor results (82,83). Interestingly, a more recent study (84) has shown the usefulness of molecular analysis, if combined with cyst fluid cytology and CEA, for identifying mucinous cysts (diagnostic accuracy of 73%) versus the use of molecular analysis alone (diagnostic accuracy of 56%). Moreover, another study also showed optimal results of molecular analysis in characterizing malignancy among mucinous cysts (85). Despite these initial results, the role of molecular analysis continues to be confined to specific experimental cases when the conventional approach is inconclusive.
Lastly, GNAS mutations (that can also be studied in pancreatic juice) seem to be the most promising molecular marker after a study that showed its presence in up to 64% of patients with IPMN (83-86). Following these results, GNAS mutations seem to be able to discriminate between IPMNs and MCAs (although their absence does not directly diagnose MCAs, as not all IPMNs express GNAS mutations) but they are not useful to detect malignancy (43). Further research is still needed in this field.
Current Guidelines and Recommendations
The first clinical guidelines for IPMNs and MCAs were published in 2006 (87) with a posterior revision in 2012 (24). These guidelines provided an essential framework for the clinical approach of both IPMNs and MCAs. However, due to the limited clinical data available, they were based on consensus agreement and lack of sufficient scientific evidence to be considered as more than expert recommendations. In fact, a recent systematic review (88) addressed the overall utility of the Sendai guidelines in the management of BD-IPMNs showing an insufficient positive predictive value ranging between 11-52% and a negative predictive value between 70-100%.
Briefly, the 2012 guidelines introduced a two-phase algorithm to predict malignancy in these lesions. It consisted of the presence of "high-risk stigmata" (obstructive jaundice in patients with cysts in the pancreatic head, enhancing intracystic solid component and a dilated MD greater than 10 mm) that straightly directed the patient to surgical resection, and "worrisome features" (cyst size greater than 3 cm, thickened/enhancing cyst walls, dilated MD of 5-9 mm, non-enhancing mural nodules, abrupt pancreatic duct caliber change with distal atrophy and lymphadenopathy) (Fig. 3), which, if present, should be further studied with EUS-FNA. If none of these features was observed, the clinical management was solely based on the cyst size. Also, these revised guidelines lowered the BD and pancreatic duct dilation threshold to 5 mm to increase the sensitivity of the diagnosis.
Since their publication, multiple studies have evaluated the guidelines accuracy. There seems to be a consensus regarding the higher accuracy of the 2012 guidelines with respect to the 2006 ones (44.8% versus 35.5%, respectively) (89). However, if the risk factors for malignancy are analyzed, the results are quite heterogeneous. Cyst size was the risk factor mostly associated with malignancy in a meta-analysis (90) that included 41 articles (3,304 resected BD-IPMNs) with a reported odds ratio of 62.4, followed by the presence of mural nodules (odds ratio 9.3). Surprisingly, a different meta-analysis (91) that reviewed surgically resected IPMNs concluded that mural nodules was the most associated risk factor for malignancy (diagnostic odds ratio 6.0) followed by MD dilation greater than 5 mm (diagnostic odds ratio 4.4). Based on their results, cyst size was not even related with malignant progression. Similarly, a recent study (92) that also analyzed a BD-IPMN surgical cohort showed the presence of mural nodules and MD dilation as the best predictors of malignancy. Cyst size greater than 3 cm seems to be the most debatable risk factor with some studies (93) supporting its role as an independent predictor of malignancy and other publications (44) recommending a conservative approach with close surveillance if present alone without other "worrisome features".
In 2015 the AGA published new guidelines for the management of PCNs (94). A significant difference regarding the previous Sendai and Fukuoka guideline was the definition of "malignancy". In these new guidelines, only studies that reported the presence of invasive adenocarcinoma were considered as malignant and, therefore, included, whereas HGD lesions were not entered in the analysis. Thereby, the AGA guidelines aimed to detect "late neoplasia" instead of incipient malignant lesions as in the 2006 and 2012 guidelines. From a clinical point of view, it seems that targeting "late neoplasia" as the diagnostic threshold may not seem the best approach for the patient due to the high lethality of these lesions once malignancy arises. In view of this, a recent study (95) aimed to assess the accuracy of the AGA guidelines in detecting advanced neoplasia. To do so, 225 patients with a PCL who underwent EUS-FNA were analyzed. Interestingly, they concluded that the AGA guidelines were inaccurate to detect PCLs with advanced neoplasia, reporting a sensitivity of 62%, a specificity of 79%, a positive predictive value of 57% and a negative predictive value of 82%. More importantly, and based on their results, these guidelines were not able to detect 45% of HGD or invasive carcinoma IPMNs.
Also, one of the most controversial points was the recommendation against continued surveillance of these lesions if no high-risk factors (i.e., development of a solid component, increasing size of the pancreatic duct, and/or diameter ≥ 3cm) were noticed after five years of follow-up. Despite the fact that this is the first time that the problematic issue of when to stop surveillance of low-risk lesions was addressed, the poor evidence that is currently available limits its generalizability. Moreover, current literature shows contradictory results regarding this matter, with several studies that analyzed BD-IPMNs reporting the development of malignancy after the initial five year period (28,29,96) whereas other publications showed a trend towards stability once the initial surveillance period has passed (97,98). A recent study involving several institutions analyzed 310 patients with asymptomatic PCLs (99). These patients were followed-up for five years or more resulting in a total of 3 (1%) patients developing malignancy. Patients with 0, 1 or 2 of the previously mentioned high risk factors had a 0%, 1% and 15% of risk of developing cancer, supporting current AGA guidelines recommendations for discontinuation of surveillance in selected low-risk patients. Moreover, a study recently conducted by the Mayo Clinic divided PCLs into Fukuoka positive or negative groups depending on the presence or absence of "high-risk stigmata/worrisome features" respectively (100). These cysts were followed-up for a median time of 4.2 years concluding that, among the Fukuoka negative cysts, the 5-year pancreatic cancer risk was 2-3% (0-2% if the malignant cases diagnosed within the six first months of follow-up were excluded). Based on their results, they suggested that surveillance after six months could be done at less frequent intervals than those exposed in the Fukuoka guidelines, supporting the trend exposed in the AGA guidelines towards a more conservative management approach.
Conclusions
PCNs and, more specifically, IPMNs and MCAs are being increasingly diagnosed in daily practice. Most of these lesions are identified during an imaging study performed for non-pancreatic reasons and, in many cases, that study is unable to provide the necessary information to decide the management approach. Combining a radiologic study with EUS-FNA and serum/tumor markers contributes to characterize and discriminate the lesion. Despite this, there are still false positives that wrongly direct the patient to surgical resection with the corresponding comorbidities and secondary effects associated.
Current guidelines lack enough long-term evidence to define a clear management strategy and, thereby, their indications are considered as just expert recommendations. Overall, there is an increased trend towards a more conservative approach of low-risk lesions. Nevertheless, further research is still needed to clearly understand the natural history of PCNs, and more specifically BD-IPMNs.
References
1. Kimura W, Nagai H, Kuroda A, et al. Analysis of small cystic lesions of the pancreas. Int J Pancreatol 1995;18:197-206. [ Links ]
2. Zhang XM, Mitchell DG, Dohke M, et al. Pancreatic cysts: Depiction on single-shot fast spin-echo MR images. Radiol 2002;223:547-53. DOI: 10.1148/radiol.2232010815. [ Links ]
3. Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: Observe or operate. Ann Surg 2004;239:651-7. DOI: 10.1097/01.sla.0000124299.57430.ce. [ Links ]
4. Girometti R, Intini S, Brondani G, et al. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: Prevalence and relation with clinical and imaging features. Abdom Imaging 2011;36:196-205. DOI: 10.1007/s00261-010-9618-4. [ Links ]
5. Gaujoux S, Brennan MF, Gonen M, et al. Cystic lesions of the pancreas: Changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg 2011;212:590-600. DOI: 10.1016/j.jamcollsurg.2011.01.016. [ Links ]
6. Chung JW, Chung MJ, Park JY, et al. Clinicopathologic features and outcomes of pancreatic cysts during a 12-year period. Pancreas 2013;42:230-8. DOI: 10.1097/MPA.0b013e31826ae31a. [ Links ]
7. Freeny PC, Saunders MD. Moving beyond morphology: New insights into the characterization and management of cystic pancreatic lesions. Radiol 2014;272:345-63. DOI: 10.1148/radiol.14131126. [ Links ]
8. Moris M, Bridges MD, Pooley RA, et al. Association between advances in high-resolution cross-section imaging technologies and increase in prevalence of pancreatic cysts from 2005 to 2014. Clin Gastroenterol Hepatol 2016;14:585-93. DOI: 10.1016/j.cgh.2015.08.038. [ Links ]
9. Hoffman RL, Gates JL, Kochman ML, et al. Analysis of cyst size and tumor markers in the management of pancreatic cysts: Support for the original Sendai criteria. J Am Coll Surg 2015;220:1087-95. DOI: 10.1016/j.jamcollsurg.2015.02.013. [ Links ]
10. Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest Endosc 2016;84:81-6. DOI: 10.1016/j.gie.2015.10.030. [ Links ]
11. Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 2012;41:380-7. DOI: 10.1097/MPA.0b013e31822a27db. [ Links ]
12. Das A, Wells CD, Nguyen CC. Incidental cystic neoplasms of pancreas: What is the optimal interval of imaging surveillance? Am J Gastroenterol 2008;103:1657-62. DOI: 10.1111/j.1572-0241.2008.01893.x. [ Links ]
13. Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: A multi-institutional study of the Japan pancreas society. Pancreas 2011;40:67-71. DOI: 10.1097/MPA.0b013e3181f749d3. [ Links ]
14. Wu BU, Sampath K, Berberian CE, et al. Prediction of malignancy in cystic neo-plasms of the pancreas: A population-based cohort study. Am J Gastroenterol 2014;109:121-9. DOI: 10.1038/ajg.2013.334. [ Links ]
15. Broughton J, Lipschitz J, Cantor M, et al. Determining the natural history of pancreatic cystic neoplasms: A Manitoban cohort study. HPB (Oxford) 2016;18:383-8. DOI: 10.1016/j.hpb.2015.11.001. [ Links ]
16. Ohashi K, Tajiri H, Gondo M, et al. A case of cystadenocarcinoma of the pancreas forming bilio-pancreatic fistula. Prog Dig Endosc 1980;17:261-4. [ Links ]
17. Kloppel G, Solcia E, Longnecker DS, et al. Histological typing of tumours of the exocrine pancreas, 2nd ed. World Health Organization: international histological classification of tumours. Berlin: Springer-Verlag; 1996. [ Links ]
18. Farrell JJ, Brugge WR. Intraductal papillary mucinous tumor of the pancreas. Gastrointest Endosc 2002;55:701-14. DOI: 10.1067/mge.2002.123641. [ Links ]
19. Kang MJ, Lee KB, Jang JY, et al. Disease spectrum of intraductal papillary mucinous neoplasm with an associated invasive carcinoma invasive IPMN versus pancreatic ductal adenocarcinoma-associated IPMN. Pancreas 2013;42:1267-74. DOI: 10.1097/MPA.0b013e3182954137. [ Links ]
20. Lafemina J, Katabi N, Klimstra D, et al. Malignant progression in IPMN: A cohortanalysis of patients initially selected for resection or observation. Ann Surg Oncol 2013;20:440-7. DOI: 10.1245/s10434-012-2702-y. [ Links ]
21. Ogura T, Masuda D, Kurisu Y, et al. Potential predictors of disease progression formain-duct intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol Hepatol 2013;28:1782-6. DOI: 10.1111/jgh.12301. [ Links ]
22. Marchegiani G, Mino-Kenudson M, Sahora K, et al. IPMN involving the main pancreatic duct: Biology epidemiology, and long-term outcomes following resection. Ann Surg 2015;261:976-83. DOI: 10.1097/SLA.0000000000000813. [ Links ]
23. Nagata N, Kawazoe A, Mishima S, et al. Development of pancreatic cancer, disease-specific mortality, and all-cause mortality in patients with nonresected IPMNs: A long-term cohort study. Radiol 2016;278:125-34. DOI: 10.1148/radiol.2015150131. [ Links ]
24. Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatol 2012;12:183-97. DOI: 10.1016/j.pan.2012.04.004. [ Links ]
25. Tanaka M. Intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2014;43:1136-40. DOI: 10.1097/MPA.0000000000000233. [ Links ]
26. Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy 2010;42:1077-84. DOI: 10.1055/s-0030-1255971. [ Links ]
27. Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: A multicenter study in japan. Pancreas 2011;40:364-70. DOI: 10.1097/MPA.0b013e31820a5975. [ Links ]
28. Khannoussi W, Vullierme MP, Rebours V, et al. The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatol 2012;12:198-202. DOI: 10.1016/j.pan.2012.03.056. [ Links ]
29. Malleo G, Marchegiani G, Borin A, et al. Observational study of the incidence of pancreatic and extrapancreatic malignancies during surveillance of patients with branch-duct intraductal papillary mucinous neoplasm. Ann Surg 2015;261:984-90. DOI: 10.1097/SLA.0000000000000884. [ Links ]
30. Mino-Kenudson M, Fernández-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut 2011;60:1712-20. DOI: 10.1136/gut.2010.232272. [ Links ]
31. Nakata K, Ohuchida K, Aishima S, et al. Invasive carcinoma derived from intestinal-type intraductal papillary mucinous neoplasm is associated with minimal invasion, colloid carcinoma, and less invasive behavior, leading to a better prognosis. Pancreas 2011;40:581-7. DOI: 10.1097/MPA.0b013e318214fa86. [ Links ]
32. Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasmsof the pancreas: An updated experience. Ann Surg 2004;239:788-97. DOI: 10.1097/01.sla.0000128306.90650.aa. [ Links ]
33. Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339-49. DOI: 10.1111/j.1572-0241.2007.01516.x. [ Links ]
34. Sahani DV, Lin DJ, Venkatesan AM, et al. Multidisciplinary approach to diagnosis and management of intraductal papillary mucinous neoplasms of the pancreas. Clin Gastroenterol Hepatol 2009;7:259-69. DOI: 10.1016/j.cgh.2008.11.008. [ Links ]
35. Kitagawa Y, Unger TA, Taylor S, et al. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg 2003;7:12-8. DOI: 10.1016/S1091-255X(02)00152-X. [ Links ]
36. Telford JJ, Carr-Locke DL. The role of ERCP and pancreatoscopy in cystic and intraductal tumors. Gastrointest Endosc Clin N Am 2002;12:747-57. DOI: 10.1016/S1052-5157(02)00026-0. [ Links ]
37. Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013;11:719-30. DOI: 10.1016/j.cgh.2012.11.016. [ Links ]
38. Xiao HD, Yamaguchi H, Dias-Santagata D, et al. Molecular characteristics and biological behaviours of the oncocytic and pancreatobiliary subtypes of intraductal papillary mucinous neoplasms. J Pathol 2011;224:508-16. DOI: 10.1002/path.2875. [ Links ]
39. Hong SM, Vincent A, Kanda M, et al. Genome-wide somatic copy number alterations in low-grade PanINs and IPMNs from individuals with a family history of pancreatic cancer. Clin Cancer Res 2012;18:4303-12. DOI: 10.1158/1078-0432.CCR-12-1075. [ Links ]
40. Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011;108:21188-93. DOI: 10.1073/pnas.1118046108. [ Links ]
41. Matthaei H, Norris AL, Tsiatis AC, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2012;255:326-33. DOI: 10.1097/SLA.0b013e3182378a18. [ Links ]
42. Dal Molin M, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol 2013;20:3802-8. DOI: 10.1245/s10434-013-3096-1. [ Links ]
43. Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large singke-instituttional series. Ann Surg 2013;258:446-75. [ Links ]
44. Lawson RD, Hunt GC, Giap AQ, et al. Pancreatic cysts suspected to be branch duct intraductal papillary mucinous neoplasm without concerning features have low risk for development of pancreatic cancer. Ann Gastroenterol 2015;28:487-94. [ Links ]
45. Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surg 2012;152:S4-12. DOI: 10.1016/j.surg.2012.05.033. [ Links ]
46. Baker ML, Seeley ES, Pai R, et al. Invasive mucinous cystic neoplasms of the pancreas. Exp Mol Pathol 2012;93:345-9. DOI: 10.1016/j.yexmp.2012.07.005. [ Links ]
47. Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: Can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg 2000;231:205-12. DOI: 10.1097/00000658-200002000-00009. [ Links ]
48. Barral M, Soyer P, Dohan A, et al. Magnetic resonance imaging of cystic pancreatic lesions in adults: An update in current diagnostic features and management. Abdom Imaging 2014.39:48-65. DOI: 10.1007/s00261-013-0048-y. [ Links ]
49. Van der Gaag NA, Berkhemer OA, Sprangers MA, et al. Quality of life and functional outcome after resection of pancreatic cystic neoplasm. Pancreas 2014;43:755-61. DOI: 10.1097/MPA.0000000000000075. [ Links ]
50. Park JW, Jang JY, Kang MJ, et al. Mucinous cystic neoplasm of the pancreas: Is surgical resection recommended for all surgically fit patients? Pancreatol 2014;14:131-6. DOI: 10.1016/j.pan.2013.12.006. [ Links ]
51. Xourafas D, Tavakkoli A, Clancy TE, et al. Noninvasive intraductal papillary mucinous neoplasms and mucinous cystic neoplasms: Recurrence rates and postoperative imaging follow-up. Surg 2015;157:473-83. DOI: 10.1016/j.surg.2014.09.028. [ Links ]
52. Jiménez RE, Warshaw AL, Z'graggen K, et al. Sequential accumulation of K-ras mutations and p53 overexpression in the progression of pancreatic mucinous cystic neoplasms to malignancy. Ann Surg 1999;230:501-9. DOI: 10.1097/00000658-199910000-00006. [ Links ]
53. O'Toole D, Palazzo L, Hammel P, et al. Macrocystic pancreatic cystadenoma: The role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc 2004;59:823-9. DOI: 10.1016/S0016-5107(04)00346-3. [ Links ]
54. Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterol 2004;126:1330-6. DOI: 10.1053/j.gastro.2004.02.013. [ Links ]
55. Correa-Gallego C, Ferrone CR, Thayer SP, et al. Incidental pancreatic cysts: Do we really know what we are watching? Pancreatol 2010;10:144-50. DOI: 10.1159/000243733. [ Links ]
56. Salvia R, Malleo G, Marchegiani G, et al. Pancreatic resections for cystic neoplasms: From the surgeon's presumption to the pathologist's reality. Surg 2012;152:S135-42. [ Links ]
57. Del Chiaro M, Segersvärd R, Pozzi Mucelli R, et al. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann Surg Oncol 2014;21:1539-44. DOI: 10.1245/s10434-013-3465-9. [ Links ]
58. Goh BK, Thng CH, Tan DM, et al. Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: A single institution experience with 114 surgically treated patients. Am J Surg 2014;208:202-9. DOI: 10.1016/j.amjsurg.2013.09.031. [ Links ]
59. Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: Can CT be used for patient triage and treatment. AJR Am J Roentgenol 2000;175:99-103. DOI: 10.2214/ajr.175.1.1750099. [ Links ]
60. Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: Relative accuracy of CT and MRI. AJR Am J Roentgenol 2007;189:648-56. DOI: 10.2214/AJR.07.2365. [ Links ]
61. Waters JA, Schmidt CM, Pinchot JW, et al. CT vs MRCP: Optimal classification of IPMN type and extent. J Gastrointest Surg 2008;12:101-9. DOI: 10.1007/s11605-007-0367-9. [ Links ]
62. Lee HJ, Kim MJ, Choi JY, et al. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clin Radiol 2011;66:315-21. DOI: 10.1016/j.crad.2010.06.019. [ Links ]
63. Nougaret S, Reinhold C, Chong J, et al. Incidental pancreatic cysts: Natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. Eur Radiol 2014;24:1020-9. DOI: 10.1007/s00330-014-3112-2. [ Links ]
64. Muthusamy VR, Chandrasekhara V, Acosta RD, et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest Endosc 2016;84:1-9. DOI: 10.1016/j.gie.2016.04.014. [ Links ]
65. Suzuki R, Thosani N, Annangi S, et al. Diagnostic yield of endoscopic retrograde cholangiopancreatography-based cytology for distinguishing malignant and benign intraductal papillary mucinous neoplasm: Systematic review and meta-analysis. Dig Endosc 2014;26:586-93. DOI: 10.1111/den.12230. [ Links ]
66. Itoi T, Sofuni A, Itokawa F, et al. Initial experience of peroral pancreatoscopy combined with narrow-band imaging in the diagnosis of intraductal papillary mucinous neoplasms of the pancreas. Gastrointest Endosc 2007;66:793-7. DOI: 10.1016/j.gie.2007.03.1096. [ Links ]
67. Nagayoshi Y, Aso T, Ohtsuka T, et al. Peroral pancreatoscopy using the SpyGlass system for the assessment of intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci 2014;21:4110-7. DOI: 10.1002/jhbp.44. [ Links ]
68. Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc 2003;58:59-64. DOI: 10.1067/mge.2003.298. [ Links ]
69. Lim LG, Lakhtakia S, Ang TL, et al. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: A multicentre Asian study. Dig Dis Sci 2013;58:1751-7. DOI: 10.1007/s10620-012-2528-2. [ Links ]
70. Rogart JN, Loren DE, Singu BS, et al. Cyst wall puncture and aspiration during EUS-guided fine needle aspiration may increase the diagnostic yield of mucinous cysts of the pancreas. J Clin Gastroenterol 2011;45:164-9. DOI: 10.1097/MCG.0b013e3181eed6d2. [ Links ]
71. Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas 2013;42:717-21. DOI: 10.1097/MPA.0b013e3182883a91. [ Links ]
72. Zhong N, Zhang L, Takahashi N, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol 2012;10:192-8. DOI: 10.1016/j.cgh.2011.09.029. [ Links ]
73. Hocke M, Cui XW, Domagk D, et al. Pancreatic cystic lesions: The value of contrast-enhanced endoscopic ultrasound to influence the clinical pathway. Endosc Ultrasound 2014;3:123-30. DOI: 10.4103/2303-9027.131040. [ Links ]
74. Nakai Y, Iwashita T, Park DH, et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc 2015;81:1204-14. DOI: 10.1016/j.gie.2014.10.025. [ Links ]
75. Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatol 2013;13:48-57. DOI: 10.1016/j.pan.2012.11.313. [ Links ]
76. Pitman MB, Genevay M, Yaeger K, et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than "positive" cytology. Cancer Cytopathol 2010;118:434-40. DOI: 10.1002/cncy.20118. [ Links ]
77. Genevay M, Mino-Kenudson M, Yaeger K, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg 2011;254:977-83. DOI: 10.1097/SLA.0b013e3182383118. [ Links ]
78. Cizginer S, Turner B, Bilge AR, et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011;40:1024-8. DOI: 10.1097/MPA.0b013e31821bd62f. [ Links ]
79. Van der Waaij LA, Van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest Endosc 2005;62:383-9. DOI: 10.1016/S0016-5107(05)01581-6. [ Links ]
80. Park WG, Mascarenhas R, Palaez-Luna M, et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas 2011;40:42-5. DOI: 10.1097/MPA.0b013e3181f69f36. [ Links ]
81. Nakai Y, Iwashita T, Shinoura S, et al. Role of serial EUS-guided FNA on pancreatic cystic neoplasms: A retrospective analysis of repeat carcinoembryonic antigen measurements. Gastrointest Endosc 2016. E-pub. DOI: 10.1016/j.gie.2016.03.1500. [ Links ]
82. Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc 2009;69:1095-102. DOI: 10.1016/j.gie.2008.07.033. [ Links ]
83. Siddiqui AA, Kowalski TE, Kedika R, et al. EUS-guided pancreatic fluid aspiration for DNA analysis of KRAS and GNAS mutations for the evaluation of pancreatic cystic neoplasia: A pilot study. Gastrointest Endosc 2013;77:669-70. DOI: 10.1016/j.gie.2012.11.009. [ Links ]
84. Al-Haddad M, DeWitt J, Sherman S, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc 2014;79:79-87. DOI: 10.1016/j.gie.2013.05.026. [ Links ]
85. Al-Haddad MA, Kowalski T, Siddiqui A, et al. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy 2015;47:136-46. [ Links ]
86. Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 2013;62:1024-33. DOI: 10.1136/gutjnl-2012-302823. [ Links ]
87. Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for man-agement of intraductal papillary mucinous neoplasms and mucinous cysticneoplasms of the pancreas. Pancreatol 2006;6:17-32. DOI: 10.1159/000090023. [ Links ]
88. Goh BK, Tan DM, Ho MM, et al. Utility of the Sendai consensus guidelines forbranch-duct intraductal papillary mucinous neoplasms: A systematic review. J Gastroint Surg 2014;18:1350-7. DOI: 10.1007/s11605-014-2510-8. [ Links ]
89. Yamada S, Fujii T, Murotani K, et al. Comparison of the internationalconsensus guidelines for predicting malignancy in intraductal papillary mucinous neoplasms. Surgery 2016;159:878-84. DOI: 10.1016/j.surg.2015.08.042. [ Links ]
90. Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intra-ductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Clin Gastroenterol Hepatol 2013;11:913-21. DOI: 10.1016/j.cgh.2013.02.010. [ Links ]
91. Kim KW, Park SH, Pyo J, et al. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pan-creas: A meta-analysis. Ann Surg 2014;259:72-81. DOI: 10.1097/SLA.0b013e31829385f7. [ Links ]
92. Jang JY, Park T, Lee S, et al. Validation of international consensus guidelines forthe resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg 2014;101:686-92. DOI: 10.1002/bjs.9491. [ Links ]
93. Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreaswithout mural nodules. Pancreas 2010;39:232-6. DOI: 10.1097/MPA.0b013e3181bab60e. [ Links ]
94. Vege SS, Ziring B, Jain R, et al. American Gastroenterological Association Institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterol 2015;148:819-22. DOI: 10.1053/j.gastro.2015.01.015. [ Links ]
95. Singhi AD, Zeh HJ, Brand RE, et al. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: A clinicopathologic study of 225 patients with supporting molecular data. Gastrointest Endosc 2016;83:1107-17. DOI: 10.1016/j.gie.2015.12.009. [ Links ]
96. Tanno S, Nakano Y, Koizumi K, et al. Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas 2010;39:36-40. DOI: 10.1097/MPA.0b013e3181b91cd0. [ Links ]
97. Rautou PE, Levy P, Vullierme MP, et al. Morphologic changes in branch duct intraductal papillary mucinous neoplasms of the pancreas: A midterm follow-up study. Clin Gastroenterol Hepatol 2008;6:807-14. DOI: 10.1016/j.cgh.2007.12.021. [ Links ]
98. Crippa S, Bassil C, Salvia R, et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: A mid-term follow-up analysis. Gut. E-pub: 2016 Jan 7. DOI: 10.1136/gutjnl- 2015-310162. [ Links ]
99. Kwong WT, Hunt GC, Fehmi SM, et al. Low rates of malignancy and mortality in asymptomatic patients with suspected neoplastic pancreatic cysts beyond 5 years of surveillance. Clin Gastroenterol Hepatol 2016;14:865-71. DOI: 10.1016/j.cgh.2015.11.013. [ Links ]
100. Mukewar S, De Pretis N, Aryal-Khanal A, et al. Fukuoka criteria accurately predict risk for adverse outcomes during follow-up of pancreatic cysts presumed to be intraductal papillary mucinous neoplasms. Gut. E-pub: 2016 July 7. DOI: 10.1136/gutjnl-2016-311615. [ Links ]
![]() Correspondence:
Correspondence:
Michael B. Wallace.
Division of Gastroenterology and Hepatology.
Mayo Clinic.
4500 San Pablo Rd.
32224 Jacksonville, Florida. USA
e-mail: wallace.michael@mayo.edu
Received: 01-10-2016
Accepted: 04-10-2016











 text in
text in