My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 n.5 Madrid May. 2017
https://dx.doi.org/10.17235/reed.2017.4239/2016
CASE REPORT
Intrahepatic clear cell cholangiocarcinoma - An uncommon histologic subtype: case report and literature review
Samuel Raimundo Fernandes1, Cilénia Baldaia1, Hugo Pinto Marques2, Francisco Tortosa3 and Fernando Ramalho1
1Department of Gastroenterology and Hepatology. Hospital de Santa Maria. Centro Hospitalar Lisboa Norte. Lisbon, Portugal.
2Department of Hepatobiliary Surgery. Hospital Curry Cabral. Centro Hospitalar Lisboa Central. Lisbon, Portugal.
3Department of Anatomic Pathology. Hospital de Santa Maria. Centro Hospitalar Lisboa Norte. Lisbon, Portugal
Author contributions: Samuel Raimundo Fernandes is the corresponding author. Preparation and review of the manuscript: Cilénia Baldaia and Hugo Pinto Marques. Analysis of anatomopathologic specimens and review of the manuscript: Francisco Tortosa. Critical review of the manuscript: Fernando Ramalho.
ABSTRACT
Clear-cell cholangiocarcinoma is a very uncommon variant of cholangiocarcinoma with a largely unknown natural history and prognosis. We report a case of a 51-year-old previously healthy woman presenting with a large liver nodule found on routine imaging. Needle biopsy of the lesion suggested a non-hepatocellular carcinoma. After extensive workup for other primary neoplasms, the patient underwent a partial hepatectomy. Histopathology was compatible with a moderately differentiated clear-cell cholangiocarcinoma. There was no evidence of liver disease in the remaining tissue. The patient underwent chemotherapy and remains in clinical remission after two years.
Key words: Liver nodule. Intrahepatic cholangiocarcinoma. Clear-cell carcinoma.
Introduction
Intrahepatic cholangiocarcinoma is the second most common primary hepatic neoplasm, and is responsible for up to 20% of all deaths related to hepatobiliary malignancies (1). Most cholangiocarcinomas are adenocarcinomas histologically. Recently, a clear cell variant has been described. This histologic subtype is very rare, and so far only nine cases of intrahepatic clear cell cholangiocarcinoma have been reported (2-8). This entity is characterized by cells with a clear cytoplasm and well-defined cell membrane, residing within a highly vascularized stroma. The clear cell changes are thought to result from accumulated glycogen, phospholipids and neutral lipids, removed during conventional histological processing (3). Clear cell cholangiocarcinoma should be differentiated from other more common clear cell neoplasms such as those arising in the kidneys, lower urinary tract, skin, breast, ovaries and pancreas. In this report, we present a patient diagnosed with intrahepatic clear cell cholangiocarcinoma. The remaining liver showed no evidence of chronic disease or cirrhosis. We appraise the clinical and anatomopathologic findings, discuss treatment and prognosis, and review the available literature.
Case report
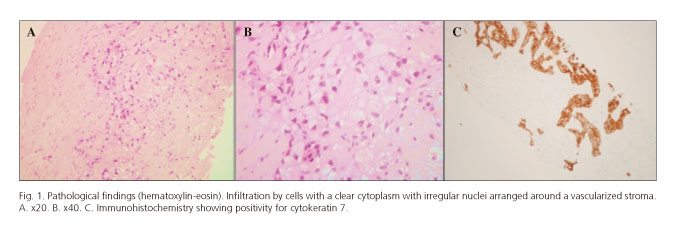
A 51-year-old black African woman was referred to our center for evaluation of a 9.5 cm solid mass in the right hepatic lobe found on an abdominal ultrasound during investigation for dyspeptic complaints. She denied any constitutional or other organ-related symptoms. Her medical history was significant for type 2 diabetes mellitus and systemic hypertension, both diagnosed seven years earlier. She was not overweight. She also denied alcohol, tobacco, and illicit drug use. There was no family history of cancer or liver disease. On physical examination, the liver was tender and extended 2 cm below the right costal margin. She had no stigma of chronic liver disease. Blood studies revealed normal aminotransferases and bilirubin with increased gamma-glutamyltranspeptidase (527 < 38 IU/L) and alkaline phosphatase (349 < 129 IU/L). Tumor markers (α-fetoprotein, CA 19.9 and CEA), auto-antibodies (anti-nuclear and anti-mytocondrial antibodies) and viral serologies (hepatitis B, C and HIV) were negative. Abdominal ultrasound and contrast enhanced CT-scan revealed a solid 9.5 x 9.8 cm lesion in the right hepatic lobe. The lesion showed heterogeneous enhancement after contrast administration. There was also potential invasion of one of the segmental branches of the portal vein. The case was discussed with our senior radiologist. As he believed there would be no further gain in diagnosis from performing a magnetic resonance imaging (MRI), ultrasound-guided fine needle aspiration of the nodule was performed. The histologic examination showed multiple nests of cells with clear vacuolated cytoplasm arranged in a dense hyalinized stroma (Fig. 1 A and B). Immunohistochemical staining showed reactivity to vimentin, epithelial membrane antigen, cytokeratin (CK) 7, 8, 18, 19, 34betaE12 and polyclonal-carcinoembryonic antigen (Fig. 1C). There was no reaction to CD10, hepatocyte antigen, glypican-3, thyroid transcription factor-1, chromogranin A, estrogen and progesterone receptors, CA-125, pS100 and CK 20. This immunohistochemical profile was incompatible with neuroendocrine and hepatocellular, thyroid, breast, ovary and kidney cancer, suggesting either a biliary or pancreatic origin. In order to exclude another primary location of the neoplasm, we performed an extensive workup including upper and lower gastrointestinal endoscopy, capsule endoscopy, breast and vaginal ultrasound, mammography and PET-scan. In light of the patient's age and good performance status, she was referred for surgery. The lesion was subsequently resected via a right hepatectomy extended to segment IV and I with resection of the portal vein. The specimen measured 11 x 10 x 9.5 cm and weighted 1,120 g. Macroscopically, the cut surface showed a solid yellowish-white tumor (Fig. 2). The tumor adhered to the gallbladder but there was a clear surgical margin. The remaining liver, hilum and supra-hepatic vessels showed no abnormalities. On histologic examination the tumor was composed of cells with a clear cytoplasm arranged in nests. Although there was evidence of microvascular invasion, there was a clear histological margin and no lymph node metastasis. The remaining liver showed no signs of chronic disease or cirrhosis. Immunohistochemical staining was positive for CK 7, focally for carcinoembryonic antigen, and negative for CK20, chromogranin, synaptophysin CD 10 and ps100. According to these findings, the tumor was classified as a moderately differentiated clear cell cholangiocarcinoma. The patient was referred for adjuvant chemotherapy with gemcitabine. After two years of follow-up, the patient remains free from disease recurrence.
Discussion
Clear cell changes have been uncommonly reported in patients with intrahepatic cholangiocarcinoma (Table I). Due to the paucity of data available, pathogenesis, risk factors and prognosis remain largely unknown. Like in other cholangiocarcinomas, an association with chronic liver disease has been suggested. This was the case in the report by Toriyama, where the patient was chronically infected with hepatitis B (2), and in the paper by Falta (5) where, like in our patient, type 2 diabetes mellitus and systemic hypertension were present, suggesting a potential link with metabolic syndrome and non-alcoholic steatohepatitis. Distinguishing clear cell cholangiocarcinoma from other benign and malignant lesions may be difficult. Considering the patient's age and gender, benign lesions including adenoma and focal nodular hyperplasia should be excluded. In addition, our patient had diabetes, a known risk factor for hepatocellular carcinoma. As the patient's radiologic findings were inconclusive, we performed a fine needle aspiration of the lesion which suggested a biliary or pancreatic origin, allowing us to exclude other more common lesions. However, as is common in this procedure, the limited sample could not provide a definite diagnosis or exclude secondary metastasis from other organs. Immunohistochemistry may play an important role in this setting. Positive expression of CK7 and the absence of CK20 are compatible with cholangiocarcinoma. On the other hand, hepatocellular carcinoma usually expresses both hepatocyte antigen and α-fetoprotein (9). Nevertheless, in many cases a definite diagnosis can only be performed post-surgically. Intrahepatic cholangiocarcinoma is an aggressive malignancy with five-year survival rates ranging between 0 and 43% (1). Chemotherapy and radiotherapy have until now shown little to no benefit in increasing survival, and surgical resection remains the only potentially curative therapy (10). Unfortunately, most cases are diagnosed at an advanced stage, when complete resection is hardly ever possible. Advanced tumor stage, positive margins after resection, and the presence of microvascular invasion or intrahepatic satellite lesions have been identified as unfavorable predictors of outcome (10). Nevertheless, some authors have suggested a more favorable prognosis in patients with clear cell changes. In the reports published to date, and including our own, nearly all patients were alive at one year, and most survived beyond three years. Once again, the number of patients and follow-up time were insufficient.
The present case seemed of great interest due to the rarity of the situation, age at presentation, and absence of other significant risk factors. We emphasize the utility of immunochemistry in reaching the final diagnosis. In conclusion, clear cell cholangiocarcinoma is a rare histologic variant. Until more data is available, we can only speculate about the risk factors, natural history and prognosis of this neoplasm.
References
1. Everhart J, Ruhl C. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterol 2009;136(4):1134-44. DOI: 10.1053/j.gastro.2009.02.038. [ Links ]
2. Toriyama E. A case of intrahepatic clear cell cholangiocarcinoma. World J Gastroenterol 2010;16(20):2571. DOI: 10.3748/wjg.v16.i20.2571. [ Links ]
3. Haas S, Gütgemann I, Wolff M, et al. Intrahepatic clear cell cholangiocarcinoma. Am J Surg Pathol 2007;31(6):902-6. DOI: 10.1097/PAS.0b013e31802c0c8a. [ Links ]
4. Tihan T, Blumgart L, Klimstra D. Clear cell papillary carcinoma of the liver: An unusual variant of peripheral cholangiocarcinoma. Hum Pathol 1998;29(2):196-200. DOI: 10.1016/S0046-8177(98)90235-0. [ Links ]
5. Falta EM, Rubin AD, Harris JA. Peripheral clear cell cholangiocarcinoma: A rare histologic variant. Am Surg 1999;65(6):592-5. [ Links ]
6. Logani S, Adsay V. Clear cell cholangiocarcinoma of the liver is a morphologically distinctive entity. Hum Pathol 1998;29(12):1548-9. DOI: 10.1016/S0046-8177(98)90031-4. [ Links ]
7. Adamek H, Spiethoff A, Kaufmann V, et al. Primary clear cell carcinoma of noncirrhotic liver: Immunohistochemical discrimination of hepatocellular and cholangiocellular origin. Dig Dis Sci 1998;43(1):33-8. DOI: 10.1023/A:1018859617522. [ Links ]
8. Albores-Saavedra J, Hoang M, Murakata L, et al. Atypical bile duct adenoma, clear cell type. Am J Surg Pathol 2001;25(7):956-60. DOI: 10.1097/00000478-200107000-00016. [ Links ]
9. Wee A. Diagnostic utility of immunohistochemistry in hepatocellular carcinoma, its variants and their mimics. Appl Immunohistochem Mol Morphol 2006;14(3):266-72. DOI: 10.1097/00129039-200609000-00003. [ Links ]
10. Mavros M, Economopoulos K, Alexiou V, et al. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma. JAMA Surg 2014;149(6):565. DOI: 10.1001/jamasurg.2013.5137. [ Links ]
![]() Correspondence:
Correspondence:
Samuel R. Fernandes.
Department of Gastroenterology and Hepatology.
Hospital de Santa Maria.
Centro Hospitalar Lisboa Norte.
Av. Prof. Egas Moniz.
1649-035 Lisbon, Portugal
e-mail: Samuelrmfernandes@gmail.com
Received: 03-02-2016
Accepted: 01-10-2016