Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.6 Madrid jun. 2017
https://dx.doi.org/10.17235/reed.2017.4701/2016
ORIGINAL PAPERS
CLIF-C ACLF score is a better mortality predictor than MELD, MELD-Na and CTP in patients with acute on chronic liver failure admitted to the ward
Rita Barosa, Lídia Roque-Ramos, Marta Patita, Gonçalo Nunes and Jorge Fonseca
Department of Gastroenterology. Hospital Garcia de Orta. Almada, Portugal
Ethics statement: This study was approved by local health research ethics boards (Garcia de Orta Ethical Committee, Garcia de Orta Centre) and the requirement for individual informed consent was waived.
Authors contributions: RB designed the study, acquired the data and drafted the manuscript. LRR, CF, JF and JF interpreted the data and critically reviewed the manuscript for important intellectual content. MP and GN collaborated in the data collection and critical review of the manuscript. All authors approved the submitted manuscript.
ABSTRACT
Background and aims: Acute-on-chronic liver failure (ACLF) is a frequent syndrome associated with high mortality. The aims of the present study are: a) comparing the Chronic Liver Failure Consortium (CLIF-C) ACLF Model for End-Stage Liver Disease (MELD), MELD Sodium (MELD-Na) and Child-Turcotte-Pugh (CTP) scores for prediction of short/medium term mortality; b) identifying ACLF prevalence in patients admitted to the ward; and c) comparing mortality between non-ACLF/ACLF.
Methods: Retrospective cohort study of 177 patients admitted to the Gastroenterology ward for acute decompensation of cirrhosis.
Results: We included 132 males. Alcohol was the cirrhosis cause/co-factor in 79.7% of cases. Infection was present in 40.7%. At admission, 19.8% of patients presented ACLF and 7.9% developed it during hospitalization (overall prevalence was 27.7%). ACLF grade 1 was diagnosed in 55.1% of the ACLF patients; grade 2, in 42.8%, and grade 3, in 2.0%. Infection (p < 0.001) and hepatic encephalopathy (p = 0.004) were more prevalent and C-reactive protein and leukocyte counts were higher in ACLF patients. ACLF 28 and 90-day mortality was 45.8% and 60.4%, respectively. The CLIF-C ACLF score was significantly superior to CTP, MELD, MELD-Na in predicting 28-day (AUROC 0.799 ± 0.078, 95% CI 0.637-0.891) and 90-day mortality (AUROC 0.828 ± 0.063, 95% CI 0.705-0.952).
Conclusion: ACLF is highly prevalent in the ward. The new CLIF scores identify high mortality cirrhotic patients admitted to the ward and are better than their predecessors to predict ACLF patients' short/medium term mortality.
Key words: Acute-on-chronic liver failure. Acute decompensation. Organ failure. Gastroenterology ward.
Introduction
Liver cirrhosis affects 0.1% of the European population and will be the 11th leading cause of death in 2030 (1,2). Acute-on-chronic liver failure (ACLF) is an evidence-based defined syndrome which is highly prevalent (3). It is characterized by acute decompensation of cirrhosis, organ failure and high short-term mortality. For the diagnosis of organ failure, the CLIF-SOFA score (Chronic Liver Failure - Sequential Organ Failure Assessment) has been used (3). Recently, two new scores were developed and validated in ACLF patients admitted to tertiary centers: a diagnostic score (Chronic Liver Failure Consortium - Organ Failure [CLIF-C OF]) and a prognostic score (Chronic Liver Failure Consortium - Acute-on-Chronic Liver Failure [CLIF-C ACLF]) (4). ACLF justifies most admissions of cirrhotic patients to intensive care units (ICU) (5). However, the acceptance of cirrhotic patients in ICU has been hampered by their high mortality, high costs per admission, and scarcity of ICU beds (5-7). As improved management of these patients led to improved outcomes in the intensive care setting, the refusal of admission of ACLF patients to an ICU may no longer be justifiable (6,8). Evidence on whom and when to transfer to an ICU is sparse, but recently it has been argued that ACLF should be managed in these units (9). Nevertheless, in view of the high prevalence of this condition and the scarcity of ICU beds, most of these patients will still be managed in a regular Gastroenterology ward.
Even though the new CLIF scores were developed and validated mainly in an ICU setting, if they prove to be better than traditional scores in predicting short and medium term mortality, they should also be systematically applied in general Gastroenterology wards. Our aims were to: a) compare the CLIF-C ACLF score with gold standard Models for End-Stage Liver Disease (MELD), MELD Sodium (MELD-Na) and Child-Turcotte-Pugh (CTP) in predicting short and medium term mortality of ACLF patients; b) identify the prevalence of ACLF, according to the CLIF-OF score, in decompensated cirrhotic patients admitted to the ward; and c) compare mortality between non-ACLF and ACLF patients (10-12).
Methods
Study design
Retrospective cohort study of patients admitted in a single non-referral center between January 2013 and September 2014.
Patients
All adult patients admitted to the ward for acute decompensation of cirrhosis, defined as gastrointestinal bleeding, acute hepatic encephalopathy, infection or ascites grade 2 or 3 from the International Ascites Club Classification developed within two weeks were included (3,13,14). Patients admitted for elective procedures, with hepatocellular carcinoma outside the Milan criteria, human immunodeficiency virus infection, other severe disease, taking immunosupressors except for corticosteroids for alcoholic hepatitis, as well as patients admitted or transferred to intensive or semi-intensive facilities were excluded. The diagnosis of cirrhosis was based on clinical, laboratory and radiological data or liver biopsy when available. Bacterial infection was defined by either the presence of neutrophils, culture or Gram stain positive for a pathogenic microorganism in normally sterile body fluid, focus of infection identified by visual inspection or other clinical or radiological evidence of infection. Fungal infections was defined by histopathologic, cytopathologic, or direct microscopic examination of a specimen, by isolation in blood cultures, or isolation from a normally sterile body fluid or site, or by serological analysis.
Patients with alcoholic hepatitis and hepatic encephalopathy and/or Maddrey discriminant function ≥ 32 were treated with prednisolone or pentoxifylline when there were contra-indications for corticosteroids.
ACLF was graded as defined by Moreau et al. (3) and the simplified CLIF-C OF score was used to define organ failure (4). Thus, hepatic failure was defined as bilirubin ≥ 12 mg/dl; renal failure, as serum creatinine ≥ 2.0 mg/dl or renal replacement therapy; hepatic encephalopathy West Haven grade 3-4 was considered to be cerebral failure; international normalized ratio (INR) ≥ 2.5 was considered as coagulation failure; circulatory CLIF-C OF sub-scores considered mean arterial pressure and circulatory failure was diagnosed when vasopressors were used, with the exception of terlipressin in hepatorenal syndrome; and, finally, respiratory failure was defined by SpO2/FiO2 ≤ 214 or the need for mechanical ventilation. ACLF grade 1 was defined by single kidney failure or other non-kidney organ failure associated with a creatinine level ranging from 1.5 to 1.9 mg/dl and/or hepatic encephalopathy West Haven grade 1-2; ACLF grade 2 was defined by two organ failures, and ACLF grade 3, by more than three organ failures as defined by the CLIF-C OF score (3). CLIF-C ACLF score was calculated using the formula previously described: 10 x (0.33 x CLIF-C OF + 0.04 x age + 0.63 x Ln [leukocyte count] -2) (4).
Vasopressors other than terlipressin for hepatorenal syndrome or octreotide for variceal bleeding, invasive mechanical ventilation and PO2/FiO2 ratio measurements were not used in the regular ward.
Short and medium term mortality were defined at 28 and 90 days, respectively.
This study was approved by the local ethic committee.
Data collection
Demographic, clinical and laboratory data were collected from each patient's clinical files. In our center, all patients were admitted to the ward from the Emergency Department, where all data required to calculate scores was systematically collected. Active alcoholism was defined as any alcohol consumption in the last three months. ACLF grade was defined in a single time point, specifically at the time of ACLF diagnosis. Survival status at 28 and 90 days was defined based on the outpatient visits following the index admission.
Statistical analyses
Normality was assessed using the Kolmogorov-Smirnov test. Mean and standard deviation or median and interquartile range were used for continuous variables. Frequencies were used for categorical variables. Continuous variables were compared using the Student's t-test or Mann-Whitney test, and the Chi-squared test or Fisher's test were used for categorical variables. Statistical analysis was performed using the SPSS software version 21 (SPSS, Chicago, IL, USA).
The performance of the CLIF-C ACLF, CTP, MELD and MELD-Na scores in predicting ACLF patients 28 and 90-day mortality was analyzed calculating the area under the receiver operating characteristics (AUROC) curves using the MedCalc software version 12.5 (MedCalc Software, Mariakerke, Belgium). These curves were compared among the different scores using the Hanley & McNeil test, and the best CLIF score cut-off to predict mortality was selected using the Youden index test. A p-value < 0.05 was considered as statistically significant.
Results
A total of 289 patients were screened. One hundred and twelve of them fulfilled at least one exclusion criteria (45 had hepatocellular carcinoma outside the Milan criteria, 37 were admitted for elective procedures, 19 had HIV infection, seven had other severe comorbidity, and four were transferred to the ICU or semi-intensive unit). Finally, 177 were enrolled, of whom one was lost at the third month of follow-up. The mean age was 60.9 years and 132 (74.6%) were male (Table I). Alcohol was the main cause of cirrhosis, being present by itself or concomitantly with hepatitis C infection in 141 patients (79.7%). In 68 patients (38.4%) the index admission was the first episode of decompensation (Table I).
At least one potential precipitating factor for decompensation was found in 146 patients (82.5%). Infection was present in 72 patients (40.7%). The most frequent infections were spontaneous bacterial peritonitis, found in 19.2% (n = 34) of the patients, urinary tract infection in 12.4% (n = 22) and pneumonia in 5.6% (n = 10). In the remaining five patients other forms of infection were found, namely candidemia or erysipelas.
Active alcoholism was present in 57 patients (32.2%). Clinical and laboratory features suggesting severe alcoholic hepatitis were found in 11 patients (6% of all patients included), eight of which had ACLF (17% of the ACLF patients). None of the patients with the diagnosis of alcoholic hepatitis underwent a liver biopsy. All 11 patients with severe alcoholic hepatitis were treated. Prednisolone was used in seven (63.7%) patients (three were non responders) and four (36.3%) were started on pentoxifylline.
None of the patients were transplanted during the 90-day period; however, ten patients (5.6%) have been transplanted at the sixth month follow-up.
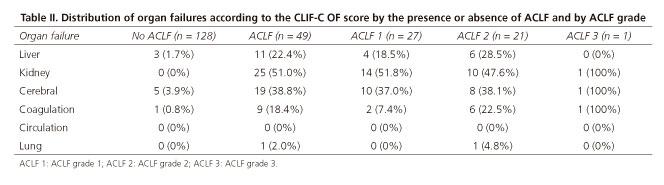
At admission, 35 patients (19.8%) had ACLF and 14 (7.9%) developed it during hospitalization. ACLF had an overall prevalence of 27.7% (49 patients). The majority of the ACLF patients presented ACLF grade 1 (n = 27, 55.1%). Furthermore, 21 (42.8%) had ACLF grade 2 and one patient (2.0%) developed ACLF grade 3. When considering ACLF patients, the most frequent organ failures were renal failure, found in 51.0%, and cerebral failure, found 38.8% (Table II).
Infection (p < 0.001) and hepatic encephalopathy (p = 0.004) were significantly more frequent in the ACLF group (Table I). The ACLF group also showed a statistic trend for less gastrointestinal hemorrhage (p = 0.057) (Table I). Sodium was significantly lower and creatinine, bilirubin and international normalized ratio were significantly higher in patients developing the syndrome compared to non-ACLF patients (Table I). C-reactive protein and leukocyte count were also higher in ACLF patients independently of the presence of infection (Table I). No significant differences were found between patients with ACLF at admission and those who developed it during hospitalization. Likewise, no predictive factors for the development of ACLF during hospitalization were found.
In patients with ACLF grade 2, the two organ failures involved the kidney, liver, central nervous system and/or lungs (Table II). Of note, 24.5% had a mean arterial pressure inferior to 65 mmHg, but it was not considered as an organ failure since vasopressor support was not started in ward.
ACLF patients had a 28-day mortality of 45.8% and a 90-day mortality of 60.4%, which was significantly higher when compared to non-ACLF cirrhotic patients (p < 0.001). Interestingly, the higher the ACLF grade, the higher the mortality (Fig. 1).
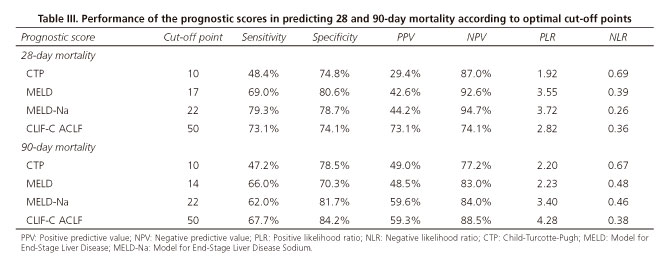
The CLIF-C ACLF score was significantly superior to CTP, MELD, MELD-Na scores to predict 28-day mortality (AUROC 0.799 ± 0.078, p < 0.05) and 90-day mortality (CLIF-C ACLF 0.828 ± 0.063, p < 0.05) (Figs. 2 and 3). The optimal cut-off point of the CLIF score selected by the Youden index was 50, with a positive predictive value (PPV) of 73.1% and a specificity of 74.1% to predict 28-day mortality, and a PPV of 59.3% with a specificity of 84.2% to predict 90-day mortality (Table III). The CLIF-C ACLF score was further explored to assess the best cut-offs to differentiate patients with the best and worst prognosis. A CLIF-C ACLF score ≤ 39 had a negative predictive value of 95.1%, with a sensitivity of 87.5% and a specificity of 53.5%, while a score ≥ 51 had a 64.0% PPV with a specificity of 93.8% and a sensitivity of 50.0% to predict short-term mortality.
Discussion
Our study confirms the high prevalence of ACLF in a general Gastroenterology ward outside a tertiary referral center. Higher proportion of identified precipitating factors, namely infection and active alcoholism, were identified in our cohort as compared with the CANONIC study (3). As those are frequently associated with ACLF, a higher prevalence of the syndrome could be expected in our cohort (3). In the same way, gastrointestinal hemorrhage was also more common in our ward but there was a trend for bleeding patients to develop ACLF less frequently, probably because they promptly seek medical care and there were major improvements in the management of this condition in recent years (15). The higher prevalence of identified precipitating factors may be related to different diagnostic procedures between the two studies, namely due to the collection of cultures, to the higher prevalence of SBP due to lower compliance to SBP prophylaxis (the most frequent infection) and to a less perfect guideline approach to variceal bleeding outside a randomized controlled trial.
The phenotype and etiology of liver disease may vary when comparing non-referral with tertiary centers, alcohol being more prevalent outside referral centers. Also, there is an important cultural variance contributing to our high prevalence of alcoholism (16,17).
Renal failure and hepatic encephalopathy (HE) were the most frequent organ failures, and HE was significantly more frequent in our cohort. The higher prevalence of infection and gastrointestinal bleeding, which are well described factors associated with the development of HE and acute kidney injury/hepatorenal syndrome, might have contributed to this difference (18,20).
ACLF grade 2 mortality in our cohort was significantly superior when compared to the CANONIC cohort. This may be related to the management of multiple organ failure in a ward, but it may be associated as well with the underestimation of the ACLF grade as the diagnosis of circulatory failure as defined in the CLIF-C OF score requires the use of vasopressors, which were not introduced in our ward patients, explaining the absence of this type of organ failure in our cohort (4). Also, there was a higher prevalence of ACLF grade 2, probably caused by an underestimation of the ACLF grade, as explained previously, or related to the poor clinical course of ACLF grade 1 patients in a ward without intensive monitoring and treatment. Moreover, there were fewer respiratory failures diagnosed in our cohort when compared to the CANONIC cohort. We could hypothesize this could be once again related to the absence of mechanical ventilation use in the ward or with the fact that data and ACLF classification were determined at admission, and respiratory failure could be a latter organ failure in patients admitted for acute decompensation of cirrhosis. Although neither mechanical ventilation nor PO2/FiO2 ratio was used in the ward, our patients had SPO2/FiO2 ratio measurements and received non-invasive ventilation as required, thus not limiting respiratory failure diagnosis.
Our work demonstrated that the CLIF-C OF and CLIF-C ACLF still have a good performance outside referral centers. Furthermore, we were able to recognize CLIF-C ACLF cut-offs that categorize patients in high and low probability of death groups. Specifically, patients with a CLIF-C ACLF score ≤ 39 had a 95.1% probability of being alive, while those with a score ≥ 51 had a 64.0% probability of dying after one month.
Moreover, the diagnosis of ACLF and the CLIF-C ACLF score are not only useful but can also be easily calculated and applied on a daily basis using the European Foundation for the Study of Chronic Liver Failure website: http://www.efclif.com/scientific-activity/score-calculators/clif-c-aclf.
Few patients were transplanted, which might be ascribed to scarcity of donors or to older age and more frequently active alcoholic consumption of our population.
The CLIF-C OF and the European Association for the Study of Liver - Chronic Liver Failure (EASL-CLIF) CANONIC definition of ACLF identified patients with high mortality admitted to the ward. Also, the CLIF-C ACLF score was significantly superior as a mortality predictor when compared to MELD, MELD-Na and CTP. Those high risk cirrhotic patients should receive close monitoring with hemodynamic and pulse oximetry surveillance, urine output, fluid balance and low threshold to initiate antibiotics. Some experts suggest that patients with a new onset elevation in creatinine, even below the 1.5 mg/dl threshold, who do not respond to the removal of risk factors and plasma volume expansion might benefit from more intensive medical treatment. Namely, vasopressors as for hepatorenal syndrome could be used, although it remains unclear if these "renal prophylactic" measures are beneficial (21). Clearly, in this setting not only organ support is important, but also prevention of further organ dysfunction.
It has been suggested that all ACLF patients should be admitted to the ICU for organ support and prevention of further organ failure (9). Refusal of cirrhotic patients with organ failure to the ICU may no longer be justifiable considering that the mortality of these patients when admitted to an ICU seems to be decreasing over the last decade (8). In fact, half of ACLF patients improve or the syndrome is resolved when best supportive care is given, and short-term mortality of ACLF patients enrolled in the CANONIC study was similar to that described for septic shock in the general population (8,9,22). However, ICU admission may not be feasible due to the high prevalence of the syndrome in a limited ICU bed availability setting. Moreover, only one third of ACLF grade 1 enrolled in the CANONIC were in the ward (3). Although we should probably aim to admit all ACLF patients to an ICU, it may be reasonable to admit some ACLF grade 1 to a ward or semi-intensive care unit and all patients with two or more organ failures to an ICU (3).
One of the limitations of our study was its retrospective nature, which could namely have limited the extensive data collection to classify organ failure. However, in our center, hemodynamics, pulse oximetry and Glasgow coma score data are systematically collected from all patients on admission. Another noteworthy limitation is the classification of the ACLF grade according to its grade at the moment of diagnosis, as prognosis has shown to correlate better with the early clinical course than with the grade of ACLF at admission (9). Limitations in the diagnosis of circulatory failure in the ward were also discussed. Nevertheless, this was the first real life study of ACLF patients admitted to the ward, describing their high mortality and validating the new CLIF scores in this setting. Indeed, the use of a partial CLIF-C OF score (excluding circulatory failure) could be considered as the CLIF-C OF score applicable to the ward, which still had a good performance and was better than its predecessors to predict short and medium term mortality.
Conclusion
This was the first real life ward study on liver cirrhosis complicated by ACLF. In our experience, ACLF presented a high prevalence (27.7%) in a regular Gastroenterology ward. ACLF patients presented higher mortality than non-ACLF cirrhotic patients. The new CLIF scores identified high mortality patients admitted to the ward, had a good performance, and were better than their predecessors MELD, MELD-Na and CTP to predict short and medium term mortality in this setting. CLIF scores routine application should be promoted to select cirrhotic patients that should be closely monitored in the ward or in a semi-intensive care unit or admitted to the ICU. Further studies are required to define which ACLF patients should be transferred to ICU in a realistic and feasible manner and when.
References
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. DOI: 10.1371/journal.pmed.0030442. [ Links ]
2. Zatoński WA, Sulkowska U, Mańczuk M, et al. Liver cirrhosis mortality in Europe, with special attention to Central and Eastern Europe. Eur Addict Res 2010;16:193-201. DOI: 10.1159/000317248. [ Links ]
3. Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterol 2013;144:1426-37. DOI: 10.1053/j.gastro.2013.02.042. [ Links ]
4. Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 2014;61:1038-47. DOI: 10.1016/j.jhep.2014.06.012. [ Links ]
5. Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatol 2011;54:1864-72. DOI: 10.1002/hep.24622. [ Links ]
6. Shawcross DL, Austin MJ, Abeles RD, et al. The impact of organ dysfunction in cirrhosis: Survival at a cost? J Hepatol 2012;56:1054-62. [ Links ]
7. Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol 2012;57:1336-48. DOI: 10.1002/hep.24622. [ Links ]
8. McPhail MJ, Shawcross DL, Abeles RD, et al. Increased survival for patients with cirrhosis and organ failure in liver intensive care and validation of the Chronic Liver Failure - Sequential Organ Failure scoring system. Clin Gastroenterol Hepatol 2015;13:1353-60. DOI: 10.1016/j.cgh.2014.08.041. [ Links ]
9. Gustot T, Fernández J, García E, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatol 2015;62:243-52. DOI: 10.1002/hep.27849. [ Links ]
10. Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. DOI: 10.1002/bjs.1800600817. [ Links ]
11. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatol 2001;33:464-70. DOI: 10.1053/jhep.2001.22172. [ Links ]
12. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018-26. DOI: 10.1056/NEJMoa0801209. [ Links ]
13. Blei AT, Córdoba J, Gastroenterology PPCotACo. Hepatic encephalopathy. Am J Gastroenterol 2001;96:1968-76. DOI: 10.1111/j.1572-0241.2001.03964.x. [ Links ]
14. Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: Report on the consensus conference of the International Ascites Club. Hepatol 2003;38:258-66. DOI: 10.1053/jhep.2003.50315. [ Links ]
15. Garcia-Tsao G, Bosch J. Varices and variceal hemorrhage in cirrhosis: A new view of an old problem. Clin Gastroenterol Hepatol 2015;13:2109-17. DOI: 10.1016/j.cgh.2015.07.012. [ Links ]
16. Thomson SJ, Berry PA, Rahman TM. The impact of organ dysfunction in cirrhosis: Survival at a cost? J Hepatol 2012;57:707-8;author reply 9. DOI: 10.1016/j.jhep.2012.03.032. [ Links ]
17. Organization WH. Global Status Report on Alcohol and Health. 2014 ed. [ Links ]
18. Strauss E, Da Costa MF. The importance of bacterial infections as precipitating factors of chronic hepatic encephalopathy in cirrhosis. Hepatogastroenterol 1998;45:900-4. [ Links ]
19. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341:403-9. DOI: 10.1056/NEJM199908053410603. [ Links ]
20. Solà E, Ginès P. Challenges and management of liver cirrhosis: Pathophysiology of renal dysfunction in cirrhosis. Dig Dis 2015;33:534-8. DOI: 10.1159/000375344. [ Links ]
21. Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531-7. DOI: 10.1136/gutjnl-2014-308874. [ Links ]
22. Gao F, Melody T, Daniels DF, et al. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: A prospective observational study. Crit Care 2005;9:764-70. DOI: 10.1186/cc3909. [ Links ]
![]() Correspondence:
Correspondence:
Rita Barosa.
Department of Gastroenterology.
Hospital Garcia de Orta.
Av. Torrado da Silva.
2801-951 Almada, Portugal
e-mail: a.rita.b@gmail.com
Received: 07-11-2016
Accepted: 10-02-2017