Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.11 Madrid Nov. 2017
https://dx.doi.org/10.17235/reed.2017.4994/2017
Recurrence of infection and diversity of Helicobacter pylori strains in an adult population in Mexico treated with empirical standard triple therapy
Recurrencia de infección y diversidad de cepas de Helicobacter pylori en adultos tratados con terapia triple estándar empírica en una población de México
Jaime Alberto Sánchez-Cuén1,3, Ana Bertha Irineo-Cabrales1,2, Nidia Maribel León-Sicairos3, Loranda Calderón-Zamora3, Luis Monroy-Higuera3 and Vicente Adrián Canizalez-Román3
1Department of Digestive Diseases. Hospital Regional del Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado. Culiacán, Sinaloa. Mexico.
2Department of Postgraduate Studies. School of Medicine. Universidad Autónoma de Sinaloa. Culiacán, Sinaloa. Mexico.
3CIASaP. School of Medicine. Universidad Autónoma de Sinaloa. Culiacán, Sinaloa. México
ABSTRACT
Background: After eradication treatment for Helicobacter pylori (H. pylori), infection could recur due to recrudescence or re-infection. The objective of this study was to determine the recurrence of H. pylori infection and identify virulent H. pylori strains one year after eradication with standard triple therapy.
Material and methods: A quasi-experimental study was performed that included a patient population with digestive diseases associated with H. pylori who had received standard triple therapy. Cultures and polymerase chain reaction (PCR) was performed on gastric biopsies for strain identification in all patients prior to eradication treatment and those with a positive carbon-14 breath test one year after eradication treatment. Statistical analysis was performed using the Student's t-test and Fisher's exact test; statistical significance was set at 0.05.
Results: One hundred and twenty-eight patients were studied, 51 (39.8%) were male and 77 (60.2%) were female with an average age of 54.8 years (DE 13.8). There was an annual recurrence of H. pylori infection in 12 (9.3%) patients. An annual re-infection and recrudescence occurred in nine (7%) and three (2.3%) patients respectively. The recrudescence rate for antigenic protein (cagA) was 1/30 (3.3%) patients and 2/112 (1.8%) patients for vacuolating cytotoxin (vacA). The re-infection rate for cagA was 3/30 (10%) patients and 6/112 (5.3%) patients for vacA.
Conclusions: The recurrence of infection in this study was higher than that recorded in developed countries with a low prevalence of H. pylori and lower than that recorded in developing countries with a higher prevalence of H. pylori. The cagA or vacA s2/m2 strains were isolated after re-infection and recrudescence.
Key words: Recurrence. Helicobacter pylori. Eradication.
RESUMEN
Introducción: posterior al tratamiento erradicador de Helicobacter pylori (H. pylori), podría presentarse recurrencia de infección debido a recrudescencia o reinfección. El objetivo de este estudio fue determinar la recurrencia de infección por H. pylori e identificar cepas virulentas de H. pylori al año posterior de su erradicación con terapia triple estándar.
Material y métodos: se realizó un estudio cuasiexperimental. La población estudiada fueron pacientes con enfermedades digestivas asociadas a H. pylori que recibieron terapia triple estándar. Todos los pacientes antes del tratamiento erradicador, y solo aquellos pacientes con prueba de aliento con carbono 14 positivo un año posterior al tratamiento se les realizaron cultivos y reacción en cadena de la polimerasa (PCR) de biopsias gástricas para identificación de cepas. Se realizó análisis estadístico mediante el test t de Student y prueba exacta de Fisher, con un nivel de significancia de 0,05.
Resultados: se revisaron 128 pacientes, 51 (39,8%) hombres y 77 (60,2%) mujeres, con una edad promedio de 54,8 (DE 13,8) años. Se halló recurrencia anual de infección por H. pylori en 12 (9,3%) pacientes y reinfección y recrudescencia anual en nueve (7%) y tres (2,3%) pacientes respectivamente. La tasa de recrudescencia en proteína antigénica (cagA) fue de 1/30 (3,3%) pacientes y en citotoxina vacuolizante (vacA) fue de 2/112 (1,8%) pacientes. La tasa de reinfección en cagA fue 3/30 (10%) pacientes y en vacA 6/112 (5,3%) pacientes.
Conclusiones: en este estudio la recurrencia de infección fue mayor que en países desarrollados con baja prevalencia de H. pylori y menor que en países en vías de desarrollo con mayor prevalencia de H. pylori. Las cepas cagA o vacA s2/m2 fueron aisladas en reinfección y recrudescencia.
Palabras clave: Recurrencia. Helicobacter pylori. Erradicación.
Introduction
Helicobacter pylori (H. pylori) is a spiral-shaped Gram-negative bacteria that colonizes the human stomach and can produce a long-term infection of the gastric mucosa (1). H. pylori infection has been present in gastroduodenal diseases, such as gastric ulcers, duodenal ulcers and gastric cancer for thousands of years (2). The gastric mucosa is colonized by H. pylori in more than 50% of the population (3). Recent studies have demonstrated a prevalence level of 70.1 and 84.7% of H. pylori infection in the wider population in Mexico (4). H. pylori is classified as a group I carcinogen by the International Agency for Research on Cancer (IARC) and is considered to be a primary factor for the development of gastric cancer (5). A sequential model has been described for preneoplastic injuries (atrophic gastritis, intestinal metaplasia and dysplasia) that range from H. pylori infection to the development of gastric cancer (6). The principal genes considered as virulence factors in H. pylori are the antigenic protein (cagA gene), the vacuolating cytotoxin (vacA gene), the promoting gene for duodenal ulcer (dupA), the gene induced through contact with the epithelium (iceA), the sialic acid-binding adhesin (sabA) and the outer inflammatory protein (gen oipA) (7). H. pylori strains, the cagA gene and more recently the vacA and iceAse genes, have been associated with a higher risk of gastric cancer (8-10).
There is still some controversy with regard to the eradication treatment for H. pylori in asymptomatic patients (11). However, there is evidence showing that the incidence and prevalence of uncomplicated peptic ulcers and the incidence of gastric cancer have decreased during recent years. This is mainly due to the availability of treatments to eradicate H. pylori in symptomatic patients (12). However, after eradication treatment, H. pylori infection could recur due to recrudescence (re-colonization by the same strain) or re-infection (colonization by a new strain) (13). The annual recurrence rates of H. pylori infection can vary from country to country (14). The recurrence of H. pylori subsequent to eradication is rare in developed countries and more frequent in developing countries. Recrudescence is thought to cause recurrence within the first 12 months after the eradication of H. pylori and re-infection can occur during the same period. The annual rate of H. pylori recurrence is 2.67% and 13% in developed and developing countries, respectively (15). Treatment with a low efficacy antibiotic increases the probability of a recrudescence of the H. pylori infection (16). Acquired immunity probably varies little from one population to the next and re-infections most likely occur in areas with a high prevalence of H. pylori infection that ultimately increase the possibility of transmission (17). A recent study indicated that the average values obtained from the post-eradication breath tests for H. pylori infection are a predictive factor for recurrence. Variables such as gender, rural and urban location, smoking habits, the eradication regimen and the method for confirming eradication have never been considered as possible predictive variables (18). In populations with a high recurrence of H. pylori infection, eradication may not be effective in the long-term and may increase the prevalence of gastric cancer and other associated pathologies. On the other hand, low rates of H. pylori infection recurrence could constitute a public health benchmark for the control of infection (19). Based on the previous, the objective of this study was to determine the recurrence of H. pylori infection and the frequency of H. pylori virulent strains isolated in adult patients treated in a specialized hospital who had undergone empirical standard triple therapy one year after eradication.
Material and methods
A longitudinal prospective quasi-experimental study was performed with a pre and post-test in one single group. The study was approved by the Commission for Research and Ethics at our hospital. The study did not violate authorship, plagiarism, conflict of interests and informed consent criteria.
The study included a population of consecutive adult patients attending the Endoscopy Service for an upper digestive videoendoscopy due to various reasons (uninvestigated dyspepsia, gastrointestinal bleeding, anemia and peptic ulcer, among others) and in whom H. pylori infection had been confirmed. None of the patients in the study were under treatment with proton-pump inhibitors (PPIs), sucralfate or antibiotics prior to H. pylori infection diagnosis by means of an endoscopy. All patients were treated with empirical standard triple therapy (40 mg omeprazole, 500 g clarithromycin and 1 g amoxicillin twice daily for two weeks). This is the current first-line regimen in Mexico, and the bacteria elimination rates are higher than 90% and 80% as calculated on an intention to treat basis, according to the recommendations of the III Mexican Consensus on H. pylori of the Mexican Gastroenterology Association (20). The study was performed from January 2014 to December 2016 at the Department of Digestive Diseases of the Regional ISSSTE Hospital in Culiacán, Sinaloa, in the North East of Mexico. Prior to eradication treatment, a histological study was performed and different types of H. pylori strains were isolated and identified (cagA and vacA genes with subtypes s1, s2, m1 and m2) in gastric samples. Eradication of the bacteria was confirmed via the carbon-14 breath test and infection was monitored during one year. This is the predetermined time-period for conducting a breath test with carbon-14. When a positive result was obtained, a new upper digestive videoendoscopy was performed in order to obtain a gastric sample and isolate and identify H. pylori strains. The inclusion criteria for patients were as follows: male and female gender; 18 to 80 years of age; eradication of H. pylori infection confirmed via a carbon-14 breath test six weeks after the eradication treatment; isolation and identification of the type of H. pylori strain prior to eradication treatment; and signed informed consent. Patients with previous recurrences of H. pylori infection were excluded, as well as patients who had not been monitored via gastroenterology appointments for one year. Patients were also excluded due to data collection errors and when the isolation and identification of the type of H. pylori strain failed in patients with a positive carbon-14 breath test after one year of monitoring.
Definition of the variables
The eradication of H. pylori infection was defined based on a negative result from the carbon-14 breath test six weeks after eradication treatment. The fact that the patient had not received PPI treatment, H2 blockers, sucralfate or antibiotics was verified. The recurrence of H. pylori infection was defined as a relapse of H. pylori infection caused by the same or a different strain (combination of the cagA and vacA genes with the s1, s2, m1 and m2 subtypes) of the bacteria after eradication using standard triple therapy. Re-infection with H. pylori was defined as an infection caused by the bacteria of a different genotype (combination of the cagA and vacA genes with the s1, s2, m1 and m2 subtypes) one year after treatment with standard triple therapy. Recrudescence of H. pylori infection was defined using a genotype identical to the strains isolated one year after eradication using standard triple therapy (cagA and vacA genes with the s1, s2, m1, and m2 subtypes) (14). The histological findings obtained from the gastric mucosa were defined according to the classification of the updated Sydney System (21).
Sample
The estimated sample size in order to detect the difference between the hypothetical and alternative proportions of 0.615 (Delta) was 126 patients. The proportion of 0.0385 for the null hypothesis was taken from a review of a study conducted in Peru (19) and the alternative value of 0.10 was used. The one-tailed Z test statistic was used. The significance level of the test was set at 0.05 with a power of 0.80. A proportion of 20% patient loss was expected. Non-probabilistic sampling was used both for convenience and for the number of consecutive patients.
Data collection
The data was collected via a primary source from the direct observation of the stomach (gastric fundus, body and antrum) from two antral gastric samples and two body samples by means of upper digestive videoendoscopy. Data was also collected from the findings of the histological study, cultures and PCR of the cagA and vacA genes from the gastric mucosa samples.
Equipment and biological samples
The upper digestive endoscopy technique and gastric biopsies
The upper digestive endoscopy was performed under intravenous sedation using an Olympus EG 29-90I videoendoscope by applying an oropharyngeal xylocaine spray in the left lateral decubitus position and the placement of the mouthpiece. The macroscopic characteristics of the stomach were recorded. Four biopsies (two from the antrum and two from the body) were taken using FB 25K-1 series biopsy forceps. Two endoscopy specialists evaluated the videoendoscopies and the endoscopic findings were defined according to the updated Sydney System (21).
Identification of the histological findings and H. pylori infection and strains
Histological study
The gastric mucosa samples (two from the antrum and two from the body) were processed using a habitual paraffin technique and histological sections were colored with hematoxylin-eosin and Giemsa stain. Complementary staining was performed using toluidine blue with a sensitivity and specificity of 96% and 99% respectively (20). The results were evaluated by two pathologists.
Carbon-14 breath test
This was performed in fasting patients who had not taken PPIs, H2 blockers, sucralfate and antibiotics in the last 30 days. In order to measure urease activity, patients ingested a urea capsule marked with 1uCi of carbon-14 for the detection of the marked carbon ten minutes later, via the analysis of the expired air with 20 ml of water. Values lower than 50 disintegrations per minute (DPM) were considered as negative for H. pylori infection, while those between 50 and 199 DPM were considered to be indeterminate, and scores greater than 200 DPM were considered as positive (19). Even though the carbon-14 breath test contains radioactive material, the radiation received by the patient is at an acceptably low level (0.03-0.3 mSv/MBq). This has not been sufficiently tested in either children or pregnant women, therefore the test was not performed in females who could be pregnant.
Isolation and identification of H. pylori strains
Bacterial culture
Two gastric samples of antral biopsies from each patient were kept in a sterile saline solution (0.9%) at 4 oC and processed for culture within two hours. The biopsies were inoculated onto the surface of Colombia chocolate agar plates enriched with Dent supplement (Oxoid®, England) containing vancomycin (5.0 mg), trimethoprim (2.5 mg), cefsulodin (2.5 mg), amphotericin B (2.5 mg) and 1% of fetal calf serum (Gibco®, USA). The biopsies were incubated in a microaerophilic atmosphere using a gas generation system that produces 5% of O2 and 14% of CO2 (CampyGen gas packs, Oxoid®, Hampshire, England) at 37 oC for three to five days. The primary H. pylori cultures were conserved at -80 oC in brain heart liquid infusion medium (BHI) with 20% of glycerol. The isolated H. pylori cultures were identified according to the morphology, Gram strain results and a positive reaction to oxidase, catalase and urease (22).
Preparation of the genomic DNA of H. pylori
Genomic DNA from H. pylori cultures was isolated using the Promega® Genomic DNA Purification Kit (Madison, Wisconsin) according to the manufacturers' instructions.
Molecular detection of H. pylori
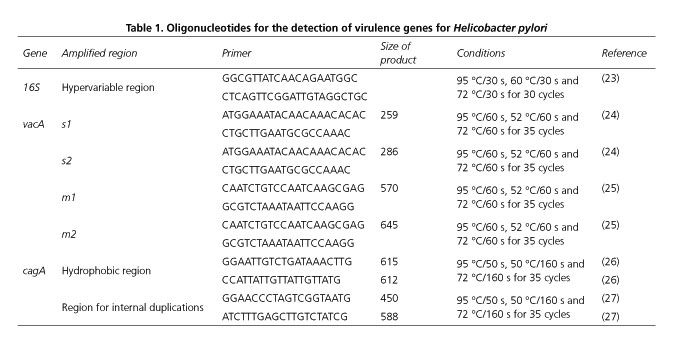
The identification of the H. pylori strains was performed by PCR using a Bio-Rad T100® thermocycler (Bio-Rad Laboratories, Hercules, California). The sequences and running conditions are shown in table 1 (23-27). When H. pylori was identified via the 16S gene, the vacA and cagA virulence genes were also detected using DNA extracted from the ATCC 26695 reference strain of H. pylori as a positive control. The PCR reaction was performed in a final volume of 25 μl with the following components: 50 mM of KCl; 20 mM of Tris HCl pH 8.4; 2.5 mM of MgCl2; 0.2 mM of each deoxynucleotide triphosphate; 1 pm/μl of each primer (Table 1); 1.25 U of Taq polymerase (InvitrogenTM, USA) and approximately 90 ng/μl of genomic DNA. The PCR amplification program was specific for each gene analyzed (Table 1) and PCR products were viewed via UV using a digital imaging system (Fotodyne, Hartland, Wi) on agarose gels at 2%.
Statistical design
The data was analyzed using the SPSS v 16 software package (SPSS, Inc., Chicago IL, USA). Average and standard deviation values were used as quantitative variables and absolute frequencies and percentages were used as categorical variables. The Student's t-test was used to compare averages and the Fisher's exact test was used to compare percentages. Statistical significance was set at 0.05.
Results
The H. pylori infection was eradicated in 142 of the 171 patients treated (83%). Four patients were excluded from the study and nine were eliminated, therefore a total of 128 patients were analyzed (Fig. 1). The average age of the patients was 54.8 years (DE 13.8), 51 years (39.8%) for males and 77 years (60.2%) for females. Twenty-nine (22.6%) patients were referred from Primary Care units and 99 (77.4%) patients came from clinical specialties.
The indications for performing an upper digestive endoscopy were uninvestigated dyspepsia in 76 (59.4%) patients, dyspepsia caused by the consumption of nonsteroidal anti-inflammatory drugs in 23 (18%), upper gastrointestinal bleeding in 14 (10.9%), iron-deficiency anemia in nine (7%), dysphagia in four (3.1%) and peptic ulcer in two (1.5%) patients. The most common histological finding was non-atrophic chronic gastritis in 114 (89.1%) patients and intestinal metaplasia was identified in 22 (17.2%) patients (Table 2).
The annual recurrence rate of H. pylori infection was 12 (9.3%) patients. Annual re-infection was identified in nine (7%) patients, while annual recrudescence was identified in three (2.3%) patients (Fig. 1). The average age of patients with and without recurrence of H. pylori infection was 60.5 years (DE 9.3) and 54.2 years (DE 14.1), respectively (p = 0.13). With regard to gender, 8/97 (8.2%) women and 4/31 (12.9%) men had a recurrence of H. pylori infection (p = 0.323).
The recrudescence rate of H. pylori infection in the cagA genotype was 1/30 (3.3%) patients, 2/112 (1.8%) for the vacA genotype, 1/15 (6.6%) for the s1/m2 genotype and 1/18 (5.5%) patients for the s2/m2 genotype. The signal sequence for type s1 was identified in 1/72 (1.4%) patients, 1/31 (3.2%) patients for type s2 and 2/33 patients (6%) for the mid-region of type m2. The rate of H. pylori re-infection due to the cagA genotype was 3/30 (10%) patients, 6/112 (5.3%) patients for the vacA genotypes, 3/18 (16.6%) patients for the s2/m2 genotype, 1/11 (9%) patients for the s1/NT genotype and 2/9 (22.2%) patients for the NT/m1 genotype. The signal sequence for type s1 was identified in 1/72 (1.4%) patients, the signal sequence for type s2 was identified in 3/31 (9.7%) patients and the signal sequence was not typed in 2/9 (22.2%) patients. The signal sequence was identified for the mid-region of the type m1 genotype in 2/68 (2.9%) patients for the mid-region of the type m2 genotype in 3/33 (9%) patients and the signal sequence for the mid-region was not typed in 1/101 (0.9%) patients (Table 3).
No significant difference were found between the frequency of H. pylori infection recurrence and the presence of the single or combined genotype (p > 0.05) (Table 4).
Discussion
The objective of the successful treatment of H. pylori infection is eradication. This benefits gastroduodenal disease patients due to lower rates of ulcer recurrence and gastrointestinal bleeding and thus changes the natural history of peptic disease. Published data suggests that H. pylori infection eradication treatment could contribute to reducing gastric cancer incidence (12,28) by modifying the progression of gastric mucosa lesions, for example via the regression of intestinal metaplasia (29). However, despite eradication treatment, H. pylori infection can recur. This is of importance as efforts to improve the prognosis of peptic disease and the incidence of gastric cancer could be unsuccessful.
Recrudescence and re-infection by H. pylori occur in different contexts, bacteria recrudescence occurs in patients that do not adhere to the eradication treatment or due to a low efficacy of the antibiotic therapy. H. pylori re-infection is related to both the high prevalence of infection in the population and environments with a greater risk of transmission. Therefore, recrudescence is a clinical problem that results from treatment failure. Re-infection is considered as a preventive medicine problem and must be treated differently.
An annual recurrence of H. pylori infection was found in 12 (9.3%) patients and an annual H. pylori re-infection in nine (7%) patients, while the annual recrudescence of H. pylori infection was identified in three (2.3%) patients. Five different types of H. pylori strains were identified according to the combination of the H. pylori genes studied. Two types of strains (with the cagA, ovacA ands2/m2 genes) were isolated in both re-infection and recrudescence, suggesting that this type of strain should receive special attention. With regard to re-infection, four different types of H. pylori strains were identified with the cagA, vacAs1, vacA s2/m2 and vacA m1 gene combinations. With regard to recrudescence, three different types of H. pylori strains were identified with the gene combinations cagA, vacA s1/m2 and vacA s2/m2.
Previous studies on H. pylori infection recurrence have obtained different results, perhaps related to the diverse regions and prevalence of H. pylori in the different populations (Table 5). The study by Gómez Rodríguez BJ et al. (18) in Spain found a 6.9% rate of recurrence of H. pylori infection, which is lower than that found in this study. A prevalence rate of H. pylori infection of 60.3% had been previously recorded in Spain (30). The study by Takes S et al. (31) in Japan found an annual recurrence rate of H. pylori infection of 0.8% and the study by Zhou LY et al. (32) in China found an annual recurrence rate of H. pylori infection of 1.75%. The results from the studies in Japan (31) and China (32) were different to those found in this study. The recurrence levels of H. pylori infection were low, even though these are developed countries with a lower prevalence of H. pylori infection than those found in Mexico. Japan and China have a population prevalence for H. pylori infection of 27.5% (33) and 31.9% (34) respectively. The study by Kim SY et al. (35) in Korea found an annual recurrence of H. pylori infection of 9.3%, which is similar to the rate observed in this study. Even though Korea is a developed country, there is a high prevalence of H. pylori infection of 54.4% (36).
Studies performed in developing countries in Latin America such as the Morgan DR et al. (37) study in Chile and Colombia found an annual recurrence of H. pylori infection of 13.6% and 18.1% respectively. The Sivapalasingam S et al. (38) study in Bolivia identified a H. pylori recurrence rate of 12%. These results are different to those described in this study as recurrence rates were higher in Latin American countries, possibly due to the high prevalence of H. pylori infection of 78%, 83.1% and 80% in Chile, Colombia and Bolivia respectively (4,38,39). The abovementioned studies do not mention whether the recurrence was due to re-infection or recrudescence, and only the study by Takes S et al. (31) in Japan found an annual re-infection rate of 0.2% and an annual recrudescence rate of 0.6%.
One of the limitations of this study is the difficulty in the identification of some of the strains from gastric samples. These difficulties begin with the transport and inoculation of the gastric biopsy on the chocolate agar plates for culture. H. pylori is vulnerable to desiccation, contact with oxygen and environmental temperature. Biopsies must be stored in saline solution for a period of no more than four hours (40), which also affects the isolation of bacteria. Occasionally, the strains cannot be typed due to the variations of the signal sequence and the mid-region in various geographical areas (41), which may explain why the majority of the previously mentioned studies did not determine whether the recurrence was due to H. pylori recrudescence or re-infection.
The carbon-13 breath test was not used in this study due to a lack of equipment availability. However, the carbon-14 breath test has a high detection rate for the recurrence of H. pylori infection with a sensitivity of 96.6% and a specificity of 100% (42). Therefore, this did not affect the findings described here. Furthermore, the kappa index score of correlation between the carbon-14 breath test and the histological study was 0.56. This could be because the histological study evaluates the presence of H. pylori in the gastric biopsies and the carbon-14 breath test evaluates the complete gastric mucosa (43). It would have been interesting to compare the recurrence rate of H. pylori infection with different first and second-line eradication therapies. No association was found between the recurrence of H. pylori infection and other variables such as smoking and breath test levels, which would have provided significant evidence for this research.
This prospective study identified recurrence due to H. pylori recrudescence or re-infection using culture based techniques, and H. pylori strains were isolated and identified via PCR. Furthermore, this study also considered that the results could infer a population-type with similar characteristics.
With regard to public health, these results may contribute to reducing the population prevalence of H. pylori infection, promoting treatment adherence and the availability of effective therapies for eradication. These factors could also reduce H. pylori infection recurrence.
In conclusion, the recurrence of infection in this study in Mexico was higher than that recorded in developed countries with a low prevalence of H. pylori, and lower than that recorded in developing countries with a higher prevalence of H. pylori. Two types of H. pylori strains with cagA, ovacA and s2/m2 genes were isolated in both re-infection and recrudescence.
Acknowledgements
We would like to thank the staff of the Endoscopy Service at the Regional ISSSTE Hospital, Culiacán, and the Program for the Strengthening of Research Projects (PROFAPI/UAS 2014) at the Autonomous University of Sinaloa.
References
1. Ranjbar R, Behzadi P, Farshad S. Advances in diagnosis and treatment of Helicobacter pylori infection. Acta Microbiol Immunol Hung 2017:1-20. DOI: 10.1556/030.64.2017.008. [ Links ]
2. Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol 2014;20(18):5191-204. DOI: 10.3748/wjg.v20.i18.5191. [ Links ]
3. Ruggiero P. Helicobacter pylori and inflammation. Curr Pharm Des 2010;16(38):4225-36. DOI: 10.2174/138161210794519075. [ Links ]
4. Porras C, Nodora J, Sexton R, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control 2013;24(2):209-15. DOI: 10.1007/s10552-012-0117-5. [ Links ]
5. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum 1994;61:1-241. [ Links ]
6. Conteduca V, Sansonno D, Lauletta G, et al. H. pylori infection and gastric cancer: State of the art (review). Int J Oncol 2013;42:5-18. [ Links ]
7. Roesler BM, Rabelo-Gonçalves EM, Zeitune JM. Virulence factors of Helicobacter pylori: A review. Clin Med Insights Gastroenterol 2014;7:9-17. DOI: 10.4137/CGast.S13760. [ Links ]
8. Trujillo E, Martínez T, Bravo MM. Genotyping of Helicobacter pylori virulence factors vacA and cagA in individuals from two regions in Colombia with opposing risk for gastric cancer. Biomedica 2014;34(4):567-73. DOI: 10.7705/biomedica.v34i4.2273. [ Links ]
9. El Khadir M, Alaoui Boukhris S, Benajah DA, et al. VacA and cagA status as biomarker of two opposite end outcomes of Helicobacter pylori infection (gastric cancer and duodenal ulcer) in a Moroccan population. PLoS One 2017;12(1):e0170616. DOI: 10.1371/journal.pone.0170616. [ Links ]
10. Dabiri H, Jafari F, Baghaei K, et al. Prevalence of Helicobacter pylori vacA, cagA, cagE, oipA, iceA, babA2 and babB genotypes in Iranian dyspeptic patients. Microb Pathog 2017;105:226-30. DOI: 10.1016/j.micpath.2017.02.018. [ Links ]
11. Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014;348:g3174. DOI: 10.1136/bmj.g3174. [ Links ]
12. Milosavljevi T, Kosti-Milosavljevi M, Krsti M, et al. Epidemiological trends in stomach-related diseases. Dig Dis 2014;32(3):213-6. DOI: 10.1159/000357852. [ Links ]
13. Raymond J, Thiberge JM, Dauga C. Diagnosis of Helicobacter pylori recurrence: Relapse or reinfection? Usefulness of molecular tools. Scand J Gastroenterol 2016;51(6):672-8. DOI: 10.3109/ 00365521.2015.1132338. [ Links ]
14. Kim SY, Hyun JJ, Jung SW, et al. Helicobacter pylori recurrence after first- and second-line eradication therapy in Korea: The problem of recrudescence or reinfection. Helicobacter 2014;19(3):202-6. DOI: 10.1111/hel.12117. [ Links ]
15. Niv Y. H pylori recurrence after successful eradication. World J Gastroenterol 2008;14(10):1477-8. DOI: 10.3748/wjg.14.1477. [ Links ]
16. Gisbert JP. The recurrence of Helicobacter pylori infection: Incidence and variables influencing it. A critical review. Am J Gastroenterol 2005;100(9):2083-99. [ Links ]
17. Parsonnet J. What is the Helicobacter pylori global reinfection rate? Can J Gastroenterol 2003;17(Suppl B):46B-8B. DOI: 10.1155/2003/567816. [ Links ]
18. Gómez Rodríguez BJ, Rojas Feria M, García Montes MJ, et al. Incidence and factors influencing on Helicobacter pylori infection recurrence. Rev Esp Enferm Dig 2004;96(9):620-3;424-7. DOI: 10.4321/S1130-01082004000900005. [ Links ]
19. Novoa Reyes I, Caravedo Martínez M, Huerta-Mercado Tenorio J, et al. Recurrence rate of Helicobacter pylori infection two years after successful eradication in Peruvian patients presenting with postprandial distress syndrome. Rev Gastroenterol Peru 2014;34(1):15-21. [ Links ]
20. Abdo-Francis JM, Uscanga-Domínguez LF, Sobrino-Cossio S, et al. Tercer consenso mexicano de Helicobacter pylori. Rev Gastroenterol Mex 2007;72(3):321-38. [ Links ]
21. Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20(10):1161-81. DOI: 10.1097/00000478-199610000-00001. [ Links ]
22. Sánchez-Cuén JA, Canizalez-Román VA, León-Sicairos NM, et al. Concordance among invasive diagnostic procedures for Helicobacter pylori infection in adults. Salud Publica Mex 2015;57(4):352-7. DOI: 10.21149/spm.v57i4.7579. [ Links ]
23. Shahamat M, Alavi M, Watts J, et al. Development of two PCR-based techniques for detecting helical and coccoid forms of Helicobacter pylori. J Clin Microbiol 2004;42(8):3613-9. DOI: 10.1128/JCM.42.8.3613-3619.2004. [ Links ]
24. Atherton JC, Cao P, Peek RM, et al. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem 1995;270(30):17771-7. DOI: 10.1074/jbc.270.30.17771. [ Links ]
25. Yamaoka Y, Kodama T, Kita M, et al. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter 1998;3(4):241-53. DOI: 10.1046/j.1523-5378.1998.08056.x. [ Links ]
26. Rudi J, Rudy A, Maiwald M, et al. Direct determination of Helicobacter pylori vacA genotypes and cagA gene in gastric biopsies and relationship to gastrointestinal diseases. Am J Gastroenterol 1999;94(6):1525-31. DOI: 10.1111/j.1572-0241.1999.1138_a.x. [ Links ]
27. Rudi J, Kolb C, Maiwald M, et al. Diversity of Helicobacter pylori vacA and cagA genes and relationship to vacA and cagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol 1998;36(4):944-8. [ Links ]
28. Lee YC, Lin JT. Screening and treating Helicobacter pylori infection for gastric cancer prevention on the population level. J Gastroenterol Hepatol 2017;32(6):1160-9. DOI: 10.1111/jgh.13726. [ Links ]
29. Sánchez Cuén JA, Irineo Cabrales AB, Bernal Magaña G, et al. Regresión de la metaplasia intestinal gástrica tras la erradicación de la infección por Helicobacter pylori en un hospital de México. Rev Esp Enferm Dig 2016;108(12):770-5. DOI: 10.17235/reed.2016.4194/2016. [ Links ]
30. Sánchez Ceballos F, Taxonera Samsó C, García Alonso C, et al. Prevalence of Helicobacter pylori infection in the healthy population of Madrid (Spain). Rev Esp Enferm Dig 2007;99(9):497-501. [ Links ]
31. Take S, Mizuno M, Ishiki K, et al. Reinfection rate of Helicobacter pylori after eradication treatment: A long-term prospective study in Japan. J Gastroenterol 2012;47(6):641-6. DOI: 10.1007/s00535-012-0536-9. [ Links ]
32. Zhou LY, Song ZQ, Xue Y, et al. Recurrence of Helicobacter pylori infection and the affecting factors: A follow-up study. J Dig Dis 2017;18(1):47-55. DOI: 10.1111/1751-2980.12440. [ Links ]
33. Hirayama Y, Kawai T, Otaki J, et al. Prevalence of Helicobacter pylori infection with healthy subjects in Japan. J Gastroenterol Hepatol 2014;29(Suppl 4):16-9. DOI: 10.1111/jgh.12795. [ Links ]
34. Yu X, Yang X, Yang T, et al. Decreasing prevalence of Helicobacter pylori according to birth cohorts in urban China. Turk J Gastroenterol 2017;28(2):94-7. DOI: 10.5152/tjg.2017.16557. [ Links ]
35. Kim SY, Hyun JJ, Jung SW, et al. Helicobacter pylori recurrence after first- and second-line eradication therapy in Korea: The problem of recrudescence or reinfection. Helicobacter 2014;19(3):202-6. DOI: 10.1111/hel.12117. [ Links ]
36. Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: Nationwide multicenter study over 13 years. BMC Gastroenterol 2013;13:104. DOI: 10.1186/1471-230X-13-104. [ Links ]
37. Morgan DR, Torres J, Sexton R, et al. Risk of recurrent Helicobacter pylori infection 1 year after after initial eradication therapy in 7 Latin American communities. JAMA 2013;309(6):578-86. DOI: 10.1001/jama.2013.311. [ Links ]
38. Sivapalasingam S, Rajasingham A, Macy JT, et al. Recurrence of Helicobacter pylori infection in Bolivian children and adults after a population-based "screen and treat" strategy. Helicobacter 2014;19(5):343-8. DOI: 10.1111/hel.12137. [ Links ]
39. Ortega JP, Espino A, Calvo B A, et al. Helicobacter pylori infection in symptomatic patients with benign gastroduodenal diseases. Analysis of 5.664 cases. Rev Med Chil 2010;138(5):529-35. DOI: 10.4067/S0034-98872010000500001. [ Links ]
40. Meunier O, Walter P, Chamouard P, et al. Isolation of Helicobacter pylori: Necessity of control of transport conditions. Pathol Biol (Paris) 1997;45(1):82-5. [ Links ]
41. Martínez A, González C, Kawaguchi F, et al. Helicobacter pylori: cagA analysis and vacA genotyping in Chile. Detection of a s2/m1 strain. Rev Med Chil 2001;129(10):1147-53. [ Links ]
42. Sugimoto M, Yamaoka Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch Immunol Ther Exp (Warsz) 2009;57:45-6. DOI: 10.1007/s00005-009-0007-z. [ Links ]
43. Silva R, Casanova G, Albarracín Z, et al. Prueba del aliento y hallazgos histopatológicos asociados a la infección por Helicobacter pylori. Gen 2012;66(2):93-9. [ Links ]
![]() Correspondence:
Correspondence:
Jaime Alberto Sánchez-Cuén.
Department of Digestive Diseases.
Hospital Regional ISSSTE de Culiacán.
Calzada Heroico Colegio Militar 875 Sur,
colonia 5 de Mayo.
80000 Culiacán, Sinaloa. Mexico
e-mail: sanchezcuen_jaime@hotmail.com
Received: 12-04-2017
Accepted: 17-06-2017











 texto em
texto em