Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.12 Madrid dic. 2017
https://dx.doi.org/10.17235/reed.2017.5067/2017
Localized gastrointestinal amyloidosis presenting with protein-losing enteropathy and massive hemorrhage
Bárbara Corrêa, Cristiane Kibune Nagasako, Ciro Garcia Montes, Marlone Cunha-Silva and Maria Aparecida Mesquita
Digestive Diseases Unit and Gastrocentro. State University of Campinas. Campinas, Sao Paulo. Brazil
ABSTRACT
Amyloidosis of the gastrointestinal tract is usually a systemic disease. Localized gastrointestinal amyloidosis without evidence of extraintestinal involvement or an associated plasma cell dyscrasia is uncommon and does not usually cause death. We report a case of a patient with localized gastrointestinal amyloidosis who presented with protein-losing enteropathy and a fatal upper gastrointestinal bleed.
Key words: Amyloidosis. Gastrointestinal bleeding. Protein-losing enteropathy.
Introduction
Amyloidosis includes a group of heterogeneous disorders characterized by the extracellular deposition of fibrils composed of low molecular weight subunits of several proteins (1,2). Amyloid deposition can be localized or systemic. The main types of amyloidosis are the AL (primary) and AA (secondary). Other forms include dialysis-related amyloidosis, heritable amyloidosis, age-related systemic amyloidosis (senile) and organ-specific amyloidosis. The diagnosis of amyloidosis requires histological confirmation and shows a characteristic green birefringence under cross-polarized light after Congo red staining.
Amyloidosis of the gastrointestinal (GI) tract is usually systemic. Localized GI amyloidosis without evidence of the involvement of other organs or an associated plasma cell dyscrasia is rare and does not usually result in death (2-4). We report a case of a patient with GI amyloidosis involving the stomach and small intestine who presented with protein-losing enteropathy and a massive recurrent and fatal upper gastrointestinal bleed.
Case report
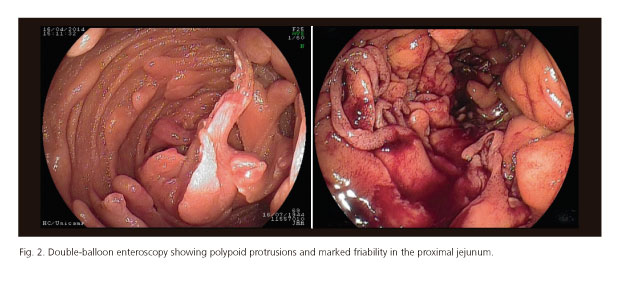
A 68-year-old man presented to our Emergency Department with acute hematemesis and melena. He reported a similar event four years previously and a diagnosis of gastric amyloidosis was made via a gastric biopsy. Clinical assessment showed that the patient was hemodynamically stable. Laboratory tests revealed a hemoglobin level of 8.0 g/dl and hematocrit of 27.9%. Upper gastrointestinal endoscopy identified irregular thickened folds associated with mucosal friability and diffuse oozing bleeding throughout the stomach. Multiple polypoid protrusions were found in the duodenum (Fig. 1). Double-balloon enteroscopy identified polypoid protrusions and marked friability in the proximal jejunum (Fig. 2). The colonoscopy was unremarkable.
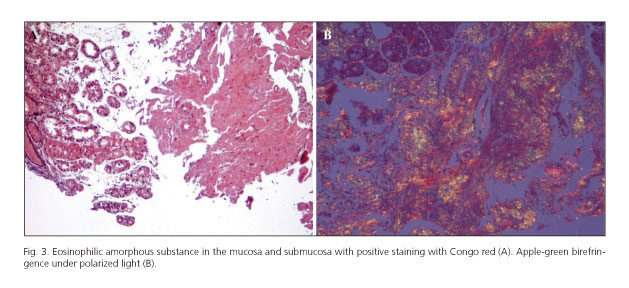
The patient was treated via a blood transfusion and an intravenous proton pump inhibitor. The bleeding stopped without a specific endoscopic intervention. Histological analysis of the biopsy specimens taken from the stomach and small bowel revealed a massive deposition of an eosinophilic amorphous substance in the mucosa and submucosa. Positive staining with Congo red and apple-green birefringence under polarized light confirmed the diagnosis of amyloidosis (Fig. 3).
The patient was referred to our outpatient clinic for further investigation. He reported symptoms of epigastric pain, postprandial gastric fullness and weight loss of 7 kg during the previous year. He also complained of diarrhea (3-4 evacuations/day, watery stools) during the last four months. There was no family history of amyloidosis and the physical examination identified edema of the lower extremities.
The coagulation profile was unremarkable. Laboratory analysis revealed profound serum hypoalbuminemia (1.6 g/dl). The urinalysis was negative for proteinuria. Rheumatic factor, antinuclear antibody, viral hepatitis panels (A, B and C) and serologic tests for HIV, toxoplasmosis and syphilis were all negative. There was no physical finding or laboratory data suggestive of tuberculosis, leprosy or other primary disorders.
Echocardiography was negative for typical amyloidosis and no abnormalities were found via cardiac magnetic resonance imaging (MRI). Gammaglobulin levels were within the normal range. Serum and urine immunoelectrophoresis showed normal levels of the kappa and lambda light chains. A bone marrow biopsy did not identify any abnormalities, and a biopsy of abdominal subcutaneous fat was also negative for amyloidosis.
Microscopic examination of a stool specimen stained with Sudan III showed steatorrhea with no evidence of parasites. The patient underwent therapy with antibiotics (metronidazole) in order to treat a potential bacterial overgrowth; however, the diarrhea persisted. The association of diarrhea, edema and hypoalbuminemia without proteinuria was suggestive of protein-losing enteropathy. This was confirmed by an accumulation of radioactivity in the bowel (jejunum and ileum) via technetium 99m-labeled albumin scintigraphy.
The patient was subsequently hospitalized in order to improve his nutritional status. He was intolerant to enteral nutrition, therefore parenteral nutrition was initiated. Ten days after admission and three months after the initial bleeding event, he presented a massive diffuse gastric hemorrhage. Endoscopic treatment with argon plasma coagulation to control bleeding was not effective. Despite the transfusion of several units of red blood cells and resuscitative treatments, the hemodynamics did not improve. The patient developed sepsis and died a few days later.
Discussion
Endoscopic findings in our patient included irregular thickened folds in the stomach and polypoid protrusions in the duodenum and jejunum. Histologic examination identified amyloid deposition in the mucosa and submucosa. This was in agreement with previous observations that showed that the thickened mucosa, nodules and polyps are associated with submucosal deposits (5).
Several factors indicated the diagnosis of localized GI amyloidosis. Firstly, there was no clinical evidence of systemic involvement during the four year period after the initial diagnosis of gastric amyloidosis. Furthermore, the abdominal fat biopsy was negative for amyloid deposition. He also had no family history of amyloidosis and there was no evidence of chronic diseases or underlying plasma cell dyscrasia.
Localized amyloidosis of the GI tract is uncommon. In a retrospective review of 2,334 patients with amyloidosis that were evaluated at a single referral center over a 13-year period, 3.3% of cases had a biopsy-proven involvement of the GI tract (6). Of these, only 21% had amyloidosis restricted to the GI tract.
The main manifestations of GI amyloidosis are bleeding, malabsorption, protein-losing gastroenteropathy and gastrointestinal dysmotility (2,3,7). GI bleeding was reported in 18% of cases in a series of 100 patients with amyloidosis (8) and in 50% of those with localized GI involvement (6). The cause of bleeding may be ischemia or infarction, vascular friability or mucosal lesions. However, bleeding may be due to generalized oozing without an identifiable source and no evidence of coagulation disturbances, as seen in our patient (3). Our patient experienced a recurrent massive and fatal bleeding episode approximately three months after the initial event and, showing that, although unusual, GI hemorrhage may be a cause of death in amyloidosis. This evolution is different to most cases reported in the literature that have a good prognosis after a GI bleed in amyloidosis (6).
Our patient also complained of diarrhea. This may be explained by several mechanisms, including a rapid transit associated with autonomic dysfunction, bacterial overgrowth and protein-losing gastroenteropathy. In the present case, intestinal protein loss might explain the presence of hypoalbuminemia and diarrhea. It is important to note that the diagnosis of protein-losing gastroenteropathy requires a clinical suspicion, as hypoalbuminemia is usually associated with proteinuria. Although there is no established treatment for this complication, a few reports have described a successful treatment with parenteral nutrition, steroids and octreotide (9,10).
In conclusion, we report the case of a patient with localized gastrointestinal amyloidosis who presented with both gastrointestinal bleeding and protein losing enteropathy. In patients with an unexplained upper GI bleed, staining for amyloid in gastrointestinal biopsies should be considered. GI bleeding in localized amyloidosis may recur and be fatal.
References
1. Pepys MB. Amyloidosis. Annu Rev Med 2006;57:223-41. DOI: 10.1146/annurev.med.57.121304.131243. [ Links ]
2. Syed U, Ching Companioni RA, Alkhawam H, et al. Amyloidosis of the gastrointestinal tract and the liver: Clinical context, diagnosis and management. Eur J Gastroenterol Hepatol 2016;28:1109-21. DOI: 10.1097/MEG.0000000000000695. [ Links ]
3. Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol 2008;103:776-87. DOI: 10.1111/j.1572-0241. 2007.01669.x. [ Links ]
4. Sattianayagam PT, Hawkins PN, Gillmore JD. Systemic amyloidosis and the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 2009;6:608-17. DOI: 10.1038/nrgastro.2009.147. [ Links ]
5. Petre S, Shah IA, Gilani N. Review article: Gastrointestinal amyloidosis - Clinical features, diagnosis and therapy. Aliment Pharmacol Ther 2008;27:1006-16. DOI: 10.1111/j.1365-2036.2008.03682.x. [ Links ]
6. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: A 13-year, single-center, referral experience. Haematologica 2013;98:141-6. DOI: 10.3324/haematol.2012.068155. [ Links ]
7. Yood RA, Skinner M, Rubinow A, et al. Bleeding manifestations in 100 patients with amyloidosis. JAMA 1983;249:1322-4. DOI: 10.1001/jama.1983.03330340064034. [ Links ]
8. Levy DJ, Franklin GO, Rosenthal WS. Gastrointestinal bleeding and amyloidosis. Am J Gastroenterol 1982;77:422-6. [ Links ]
9. Shin JK, Jung YH, Bae MN, et al. Successful treatment of protein-losing enteropathy due to AA amyloidosis with octreotide in a patient with rheumatoid arthritis. Mod Rheumatol 2013;23:406-11. DOI: 10.3109/s10165-012-0675-0. [ Links ]
10. Fushimi T, Takahashi Y, Kashima Y, et al. Severe protein losing enteropathy with intractable diarrhea due to systemic AA amyloidosis, successfully treated with corticosteroid and octreotide. Amyloid 2005;12:48-53. DOI: 10.1080/13506120500032725. [ Links ]
![]() Correspondence:
Correspondence:
Maria Aparecida Mesquita.
Department of Digestive Diseases.
Department of Clinical Medicine.
State University of Campinas.
Cidade Universitária Zeferino Vaz.
Rua Tessália Vieira de Camargo, 126.
13083-887 Campinas, Sao Paulo. Brazil
e-mail: m.aparecida.mesquita@gmail.com
Received: 22-05-2017
Accepted: 12-09-2017