INTRODUCTION
Obscure gastrointestinal bleeding (OGIB) represents approximately 5% of all gastrointestinal bleeding episodes and is defined as a bleed of unknown origin that persists or recurs after an initial negative evaluation including esophagogastroduodenoscopy and ileocolonoscopy 1) (2) (3. Previous studies have shown that the source of the bleed responsible for OGIB is usually located in the small bowel 2) (1) (4. Possible small bowel etiologies of OGIB include angioectasias, erosions and ulcers secondary to Crohn's disease or non-steroidal anti-inflammatory drug use, polyps, tumors, Meckel's diverticulum, varices and Dieulafoy's lesions 5) (6) (7) (8. In Western countries, angioectasias represents the most frequent lesion found during device assisted-enteroscopy (DAE) in patients with OGIB, which is the main indication for this technique 6) (9) (10) (11) (12).(3) (6) (8 Moreover, the rebleeding rate in OGIB is significantly higher in patients with small bowel vascular lesions compared to other small bowel lesions 9) (10. Even though the development of capsule endoscopy (CE) and DAE has led to an increased knowledge of angioectasias located in the small bowel, little is known about the efficacy endoscopic treatment in these lesions 9) (10) (13) (14. The global rebleeding rate of small bowel vascular lesions (SBVL) after one endoscopic therapeutic session with DAE is 40% 9. The current data with regard to the efficacy of further endoscopic therapy for the reduction of the rebleeding rate of previously treated SBVL is limited 15. The aim of this study was to evaluate the rebleeding rate of angioectasias after a second endoscopic treatment with balloon-assisted enteroscopy (BAE) in patients that experienced a rebleeding episode after the first endoscopic treatment.
METHODS
Patients
Patients who underwent a second DAE for endoscopic therapy of small bowel angioectasias between May 2005 and August 2015 at the Gastroenterology departments of the Centro Hospitalar Vila Nova de Gaia/Espinho (CHVNG/E) and Morales Meseguer Hospital (MMH) were identified from a prospective database. Patients were included in the study if they had an angioectasias associated rebleeding episode after a first endoscopic treatment and underwent a second endoscopic therapy with BAE. Exclusion criteria included any patient less than 18 years, pregnant women, a lack of informed consent and/or the presence of concomitant gastrointestinal lesions which explained the gastrointestinal bleed.
The medical history of patients was reviewed and data with regard to demographics, past medical and surgical history, medications and characteristics of the first rebleeding episode of SBVL were extracted. Moreover, information with regard to the first and second BAE and other diagnostic and therapeutic approaches after the first BAE was also collected. Patients were followed-up until a recurrence of gastrointestinal bleeding after a second endoscopic therapy session or until the end of the follow up period defined as August 2015. Informed consent was obtained from all patients and the study protocol was approved by the ethics committee.
Enteroscopy procedures
The standard push and pull technique was used in all procedures at both centers. A single-balloon enteroscope (SIF-Q180, Olympus, Tokyo, Japan) was used in procedures performed at the CHVNG/E and a double-balloon enteroscope (EN-450T5, EN-450P5 and EN-580T; Fujinon Inc., Saitama, Japan) was used at MMH. These enteroscopes were 200 cm long and had working channels ranging from 2.2 to 3.2 mm that enabled the use of most diagnostic and therapeutic accessories.
An overnight fast was required for antegrade procedures and bowel preparation with 4 L of polyethylene glycol solution the day before the procedure was required for retrograde BAE. Patients underwent deep propofol sedation and were positioned in the left lateral decubitus. All procedures were performed without fluoroscopic control by endoscopists with a wide experience in DAE (RP with AR at CHVNG/E and EPCM at MMH).
Procedural data and events were collected in a prospective database at both the CHVNG/E and MMH. All enteroscopy reports and their respective iconography data were reviewed by the authors (AP, RP, EPCR and EPCM). Angioectasias found and treated during all BAE procedures were classified according to the Yamamoto classification 16. A long hospitalization period (> 5 days), acute pancreatitis, bleeding with a requirement of a blood transfusion or perforation were defined as major adverse events. Enteroscopy-related variables including the type of BAE used, the depth and route of insertion, the endoscopic findings, therapeutic procedures and possible complications were recorded.
Hemostatic therapies
Conventional argon plasma coagulation (APC) probes that are traditionally used for colonoscopy were used at both centers. The APC 300/ICC 200 (ERBE Elektromedizin, Tübingen, Germany) with an argon flow rate set at 1.0-1.2 l/min and a power output at 20-40 W was used at both centers. Submucosal adrenaline (1/10000) injection (either for lifting or hemostasis) and conventional hemostatic clips (Resolution Clip, Boston Scientific) were used whenever appropriate.
Outcome
The outcome was defined as the recurrence of gastrointestinal bleeding after a second endoscopic therapy session, which included the following: a) the need for a blood transfusion; b) the presence of overt bleeding (melena, hematemesis or hematochezia); and c) a decrease in hemoglobin of greater than 2 g/dL, after the exclusion of all other causes of anemia, as reported previously 10.3 The first rebleeding episode was defined in the same manner.
Statistical analysis
Descriptive results are presented as percentages, medians and interquartile range (IQR). Kaplan-Meier survival analysis was used to estimate the non-bleeding time which was defined as the time from the second endoscopic treatment and the recurrence of a SBVL bleed, as previously described. The statistical significance level was set at p < 0.05. The Statistical Package for Social Sciences version 20.0 (IBM Corp., Armonk, New York, USA) was used for data entry and data analysis.
RESULTS
Patients and clinical characteristics
Thirty-seven patients underwent a second endoscopic treatment with BAE after experiencing a rebleed of angioectasias despite a first endoscopic treatment between May 2005 and August 2015. Most patients were male (67.7%; n = 25) with a median age of 70 years (IQR 66 -75). Twenty-two of 37 (59.5%) patients had cardiac disease, 9/37 (24.3%) had aortic valve stenosis, 10/37 (27%) had chronic renal disease and 6/37 (16.2%) had chronic liver disease (Table 1). Almost half of the patients (45.9%) were taking antiplatelet drugs and 11/37 (29.7%) were anticoagulated.
Table 1 Characteristics of patients with angioectasias that underwent endoscopic treatment with balloon-assisted enteroscopy

ASA: acetylsalicylic acid; IQR: interquartile range; NSAIDs: nonsteroidal anti-inflammatory drugs; OGIB: obscure gastrointestinal bleeding.
Rebleeding after the first endoscopic session manifested as a drop in the hemoglobin level of > 2 g/dL in 18/37 (48.6%) patients, the need for a blood transfusion in 11/37 (29.7%) patients (with or without a hemoglobin drop of > 2 g/dL) and as overt bleeding (with or without a hemoglobin drop) and/or the need for a blood transfusion in the remaining 8/37 (21.6%) patients. In addition to the DAE for the investigation of the rebleeding event, 12/37 (32.4%) patients underwent upper endoscopy, 3/37 (8.1%) patients underwent colonoscopy and 12/37 (32.4%) patients had a repeat CE.
Deep enteroscopy procedures
First device assisted-enteroscopy
The first DAE was usually performed via an oral approach in 29/37 (78.4%) patients (Table 2). The majority of angioectasias identified (51.4%; n = 19) were classified as type 1a according to the Yamamoto's Classification 16. Moreover, these lesions were more commonly located in the jejunum (43.2%; n = 16), with a median of 4 (IQR 1.5-8.5) angioectasias per examination. APC was performed in all cases, either as a single hemostatic modality in 83.8% (n = 31) of the procedures or combined with a clip or adrenaline in the remaining six procedures.
Second device-assisted enteroscopy
The oral approach was used in 30/37 (81.1%) cases and 7 (18.9%) underwent a complete examination (Table 2). Angioectasias Type 1b according to the Yamamoto Classification 16 were identified in 54.1% (n = 20) of procedures. These lesions were mainly located in the jejunum (48.6%; n = 18) and multiple lesions were frequently found, with a median of 3.0 (IQR 1.0-5.0) angioectasias per examination. APC was employed as a single therapy in 81.1% (n = 30) of procedures and complemented with clips and/or adrenaline in the remaining 18.9% (n = 7) of procedures. There were no reported minor or major complications during or after the procedure.
Rebleeding
Fifteen of the 37 patients (40.5%) that underwent a second endoscopic therapy experienced rebleeding. Rebleeding occurred as an overt gastrointestinal bleed in 7 (43.8%) patients, as a decrease of greater than 2 g/dL in the hemoglobin level in 5 (31.3%) cases and as the need for a blood transfusion in 3 (18.8%) cases. Kaplan-Meier curve analysis (Fig. 1) showed that the majority of the rebleeding episodes occurred within the first 12 months of follow-up, resulting in a rebleed rate of 33.1% at 6 months, 39.1% at 12 months and 52.6% at 24 months.
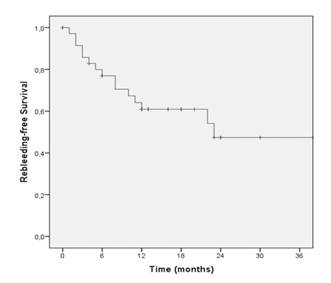
Fig. 1 Kaplan-Meier curve depicting rebleeding during follow-up. The majority of the rebleeding episodes occurred during the first 12 months of follow-up, resulting in a rebleeding rate of 33.1% at 6 months, 39.1% at 12 months and 52.6% at 24 months.
All rebleeding events occurred within the first 24 months of follow-up and the last rebleeding event occurred after 23 months of follow-up, whereas after 24 months of follow-up, only 5 patients without a rebleed event maintained a follow-up for a maximum period of 44 months. None of these 5 patients re-bled during this follow-up period. None of the variables analyzed including age, gender, medical comorbidities, past abdominal surgery, medications, type of first rebleeding episode and characteristics of the first and second DAE were associated with a significantly higher risk of rebleeding after two endoscopic therapy sessions according to univariate analysis.
DISCUSSION
In Western countries, SBVL represent the most frequent small bowel finding in patients with OGIB 10. 3 The exact prevalence of angioectasias is unclear because most patients are asymptomatic and only 10% of all patients will experience a symptomatic gastrointestinal bleed during their lifetime 13) (17) (18) .(5) (7) (11 The clinical presentation of angioectasias includes overt bleeding or iron deficiency anemia, which represent the main indications for endoscopic treatment 3) (13) (19) (20) (5) (10 Nevertheless, recurrence rates after medical, endoscopic and/or surgical therapy of SBVL remain unclear 10) (13. A recent study revealed that the global rebleeding rate after one session of endoscopic treatment of SBVL is 40% and rebleeding after two years of follow-up occurs in 38.2% of patients 9. Moreover 12, two recent systematic reviews revealed a rebleeding rate of 45% in patients with a mean follow-up time of 26 ± 15 months and 42.7% during 1.5 to 2 years 13) (17 It should be noted that most studies only evaluated rebleeding rates of SBVL after one endoscopic session and data with regard to the outcome of additional endoscopic sessions of SBVL are 5) (7 very scarce.
In this study, the global rebleeding was 40.5% in patients with angioectasias who underwent a second endoscopic session with DAE. Moreover, most rebleeding episodes (13/15, 86.7%) occurred during the first 12 months of follow-up. In spite of the high absolute rebleeding rate detected shortly after endoscopic therapy, a relative effective reduction of rebleeding occurred in a subset of patients. In this sense, there are a high proportion of patients that presented with angioectasia-related OGIB who will need several DAE procedures during their natural history. However, the goal in this selected group of patients with recurrent OGIB episodes should also include a decrease in transfusion requirements and the stabilization of hemoglobin levels as well as the reduction of the absolute rebleed rate, as previously demonstrated in treatment-naïve patients 9. Most of these patients have many comorbidities and severe acute OGIB episodes which may be avoided or minimized with an adequate endoscopic treatment and follow-up. Other reports suggest a reduction in rebleeding rates in patients who received additional therapy. This is probably as a result of intensive enteroscopy and increased complete enteroscopy rates, which may allow the treatment of SBVL that were not reached in previous examinations as there were multiple lesions occurring throughout the gastrointestinal tract 15) (18) (21) (22.
Furthermore, the rebleeding rate of the second endoscopic treatment observed at 12 months (39.1%) was similar to the rebleeding rate at 24 months previously reported in studies of naïve patients 9) (13) (17. The reason for the increased early rebleeding rate in patients who have previously re-bled after a first endoscopic treatment is unknown. In addition to treatment inefficacy and the multiplicity of small-bowel angioectasias, some of which may be missed, this group of patients may be more prone to bleed.
Alternative treatment modalities for angioectasias include medical treatment or surgery. Studies regarding the use of medical therapy are limited and comprise a small number of patients 3) (13. 5) 10)Recent evidence failed to confirm the efficacy of treatment with estrogens and progesterone 3) (13. 5) (10On the contrary, somatostatin analogues and thalidomide showed a reduction in bleeding rates and the need for blood transfusions 3) (13. 5) (10However, the use of thalidomide may be limited due to the significant side effects on the central nervous system. Nowadays, the use of intraoperative enteroscopy and enterectomy for the treatment of angioectasias has been replaced by CE and DAE 13) (23. 5 As both endoscopic and medical therapies are still used in clinical practice for SBVL, the cornerstone of endoscopic treatment may encompass a strict selection of patients and lesions characteristics 3. Future studies are needed to compare different approaches, including repeated endoscopic therapy sessions or a combination of endoscopic treatment and medical therapy 10) (21.
The present study has potential limitations, including its retrospective nature and the small number of cases. However, studies evaluating rebleeding after a first endoscopic treatment are also based on small patient groups.
In conclusion, despite the high absolute rebleeding rate detected shortly after endoscopic therapy, further endoscopic sessions for angioectasias may be beneficial due to the relative effective reduction of rebleeding in a sub-group of patients.














