INTRODUCTION
Barrett's esophagus (BE) is a metaplastic or abnormal change in the cells that line the lower portion of the esophagus, resulting in the replacement of normal stratified squamous epithelium with simple columnar epithelium with globet cells 1. The definition and diagnostic criteria of BE and the histopathological stage classification is difficult and varies worldwide 2) (3. However, there is a majority agreement that a pathology report should state the presence or absence of intestinal metaplasia (IM) as well as the endoscopic presence of columnar mucosa of the esophagus 2. Malignant degeneration is thought to occur via a multistep morphological pathway from IM to low-grade dysplasia (LGD), high-grade dysplasia (HGD) and eventually esophageal adenocarcinoma (EAC) 2. Once established, BE does not regress despite the control of gastroesophageal reflux disease symptoms 4 and is considered to be a precancerous condition. The evolutionary stages of the disease may provide an opportunity to intervene prior to the development of EAC 5.
The risk of progression of BE within the different stages is also difficult to accurately predict 2) (3) (6) (7. In general, the risk of progression to EAC is lower in BE patients with IM and increases progressively in BE patients with LGD and HGD 2) (3) (6. The rate of progression of non-dysplastic Barrett esophagus (NDBE) to EAC is estimated to be between 0.1 and 0.5% per year, respectively 8) (9. However, in patients with dysplastic Barrett esophagus, the risk of progress to EAC is reported to increase to 10% per year (in patients with HGD) 10.
The choice of treatment depends on the presence and grade of dysplasia 3) (7) (10) (11) (12. Guidelines agree that endoscopic mucosal resection (EMR) is the optimum therapeutic alternative for early EAC and for HGD with visible, nodular areas. Endoscopic radiofrequency ablation (RFA) is used for flat HGD 3) (7) (10) (11) (12. and esophagectomy is reserved only for patients with EAC that extends into the submucosa 7) (11. Consensus documents also recommend endoscopic ablation for BE patients with LGD (2,3,6,7), while the optimum treatment of IM remains unclear and further research is required 2) (3) (11) (12.
BE has a significant economic impact on healthcare systems due to the high prevalence and long-term cost of disease management. The annual treatment costs for BE are estimated at 328 million for a total of 540,131 BE patients diagnosed in Spain 13. The economic burden of BE is expected to rise, due to the increasing incidence over recent decades 14. The direct costs associated with BE include the costs of the physician and hospital visits, the cost of endoscopic procedures and possible complications, such as stricture or perforation and the cost of anti-neoplastic medication for unresectable or persistent EAC 13.
There are no formal treatment guidelines for dysplasic BE intervention in Spain. According to clinical expert opinion, the most frequently employed standard of care (SoC) during recent years was esophagectomy for HGD and endoscopic surveillance for LGD. Recently, RFA has emerged as an alternative treatment for BE in Spain, although no assessment has been made of its economic value.
Due to the long-term economic challenges of BE, the uncertainty with regard to the relative value of alternative healthcare interventions for its management and budget constraints in the Spanish National Health Service (NHS); the objective of this study was to assess the cost-effectiveness of the inclusion of RFA in the SoC of dysplastic BE in Spain.
MATERIAL AND METHODS
Study design
A literature review was carried out in order to identify pharmacoeconomic analyses of dysplastic BE and obtain the main inputs of the model. A panel of three Spanish clinical experts in digestive endoscopy participated in the model design, input selection, model assumptions, reporting and interpretation of results. The study was performed by means of a structured questionnaire and telephone interviews. A final consensus on the study outcomes was agreed via a teleconference.
Patients
The study population included two hypothetical cohorts of Spanish patients diagnosed with either LGD or HGD. According to the evidence published in the main clinical trials that have evaluated RFA, the mean age of the two cohorts was set at 65 years (range 55-75 years) 1) (14) (15.
Treatment strategies
According to the clinical experts, the SoC for HGD was esophagectomy and endoscopic surveillance once per year for LGD. EMR was thought to precede RFA in 15% of patients (for visible nodular areas). For editing purposes, this procedure is referred to as endoscopic treatment based on radiofrequency ablation plus endoscopic mucosal resection (RFA-EMR) in the manuscript.
The frequency of endoscopic surveillance was agreed upon by the panel of clinical experts. The frequencies were once every 3 years for cured patients with a history of BE, once every year for patients with LGD, once every 6 months for patients with HGD and once every 3 months for patients with EAC. Patients aged > 75 years were thought to have suspended regular endoscopic surveillance due to the low cost differential between the treatment strategies assessed in the model.
Type of analysis
A cost-effectiveness model was built using Microsoft Excel 2013 to compare the costs and effects of RFA-EMR versus SoC. The cost-effectiveness of RFA-EMR compared to SoC alone for BE with HGD and LGD was assessed using the incremental cost-effectiveness ratio (ICER). Both the cost and effectiveness generated by these alternatives were compared as follows:
CostRFA-EMR and CostSoC represent the cost associated with RFA-EMR and SoC, respectively. EffectivenessRFA-EMR and EffectivenessSoC represent the clinical consequences in terms of life-years gained (LYG), quality-adjusted life years (QALY) gained and esophagectomy or EAC cases prevented. The ICER was expressed as the incremental cost per QALY gained of RFA-EMR compared to SoC. The number of patients that needed to be treated (NNT) to prevent one esophagectomy, one course of chemoradiotherapy or one case of EAC was estimated. The associated cost savings were also estimated.
The analysis was performed from a Spanish NHS perspective, considering only direct medical costs with a time horizon of 15 years. A discount rate of 3% was applied to future costs and outcomes in accordance with Spanish guidelines 16.
Pharmacoeconomic model
A semi-Markov model was used to simulate patient transitions between six health states that reflected disease progression and patient survival during the study period. Health states were defined according to the presence and degree of dysplasia as follows: cured with a history of BE (patients with neither dysplasia nor IM after successful treatment with RFA or esophagectomy), NDBE (patients without dysplasia but with IM), LGD, HGD, EAC and death. Figure 1 shows a simplified diagram of the model.

Fig. 1 Transition state diagram (NDBE: non-dysplastic Barrett esophagus; LGD: low-grade dysplasia; HGD: high-grade dysplasia; EAC: esophageal adenocarcinoma; RFA-EMR: endoscopic treatment based on radiofrequency ablation plus endoscopic mucosal resection; EMR: endoscopic mucosal resection; BE: Barrett's esophagus; CE-IM: complete eradication of intestinal metaplasia; CE-D: complete eradication of dysplasia).
The patient cohort entered the model in either the LGD or HGD health state. Depending on the transition probabilities reflected by the natural course of the disease and the efficacy of the interventions, at the end of each cycle (1 year) the patients remain in the same health state or transition to another health state. Conservatively, it was assumed that patients with a history of BE that were cured after esophagectomy would have no risk of recurrence, while patients with a history of BE that were cured after RFA would have the same disease progression rates as NDBE patients. However, more optimistic scenarios were considered after RFA. The major complications and mortality associated with the diagnosis and treatment of EAC, together with age-related mortality were also included 17.
Clinical inputs
The efficacy of the treatment strategies, defined as the complete eradication of intestinal metaplasia (CE-IM) or complete eradication of dysplasia (CE-D) and the transitions between health states, were based on previously published economic models, randomized controlled trials and systematic reviews (Table 1).
Table 1 Model inputs: efficacy and safety of treatment alternatives, transition probabilities (natural history of Barrett's esophagus) and model utilities

RFA: radiofrequency ablation; LGD: low-grade dysplasia; CE-IM: complete eradication of intestinal metaplasia; CE-D: complete eradication of dysplasia; IM: intestinal metaplasia; HGD: high-grade dysplasia; EAC: esophageal adenocarcinoma; EMR: endoscopic mucosal resection; NDBE: non-dysplastic Barrett esophagus; BE: Barrett esophagus. *It was assumed that after RFA, regardless of its success, that patients would not undergo any further RFA treatment. †CE-D with residual IM: the result of the subtraction between CE-D and CE-IM (NDBE). ‡Estimated from a difference of 100% and the probabilities of transit to other stages. §It was assumed that after esophagectomy in HGD or resectable EAC, patients would not undergo any further esophagectomy and would initiate endoscopic surveillance. ‖Range values were estimated from a 25% variation in the difference in terms of utility of consecutive health states.
Use of resources and costs
The direct medical costs considered in the model included drugs costs, procedures cost, follow-up costs and treatment complications costs. Clinical management patterns and the use of resources associated with the management of patients with BE in Spain were consulted and validated by the panel of three clinical experts. Unit costs were obtained from national healthcare cost databases 18) (19 and all costs were expressed in Euros from 2016 (Table 2).
Table 2 Model inputs: costs
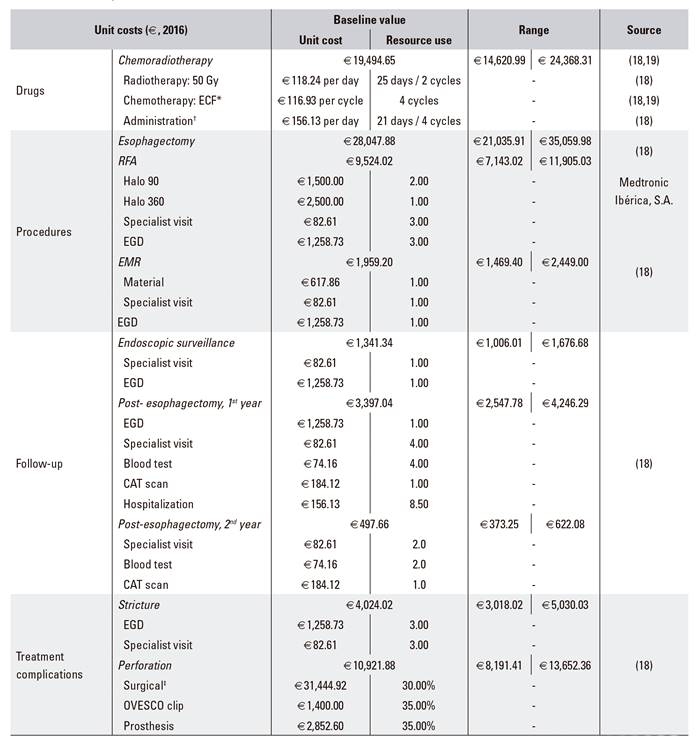
Gy: gray; RFA: radiofrequency ablation; EMR: endoscopic mucosal resection; EGD: esophagogastroduodenoscopy; CAT: computerized axial tomography. *Chemotherapy with ECF includes 50 mg/m2 on day 1 of epirubicin, plus 60 mg/m2 on day 1 of cisplatin, plus 200 mg/m2/day of 5-fluorouracil. †Oncological hospital day stay. ‡When the perforation was resolved by surgical intervention, it was assumed that an equivalent use of resources such as esophagectomy plus the 1st year of follow-up post-esophagectomy would be required.
Quality of life
Utility values were used to represent impact in terms of quality of life associated with a particular health state on a scale of 0 (death) to 1 (perfect health). The assignment of utility values to each health state of the model allowed the calculation of the QALYs associated with each alternative. As data on the quality of life were not collected in clinical trials, utility values were obtained from the literature. The model used the utility values published by Gerson et al. (2007) 20 that were adjusted to the utility of the Spanish population aged between 65-69 years (0.88) 21 (Table 1).
Sensitivity analysis
In order to assess the influence of parameter uncertainty on the results and the robustness of the model, the main assumptions of the model were varied over a wide range of values in a series of one-way sensitivity analyses (OWSA). The horizon modelled was varied between 5 and 25 years and the discount rate applied to costs and effects varied between 0% and 5%. The ranges used for the remaining parameters were based on published data. In the absence of published data, baseline rates were increased and decreased by 25% and were adjusted according to the consensus of the clinical expert panel.
A structural sensitivity analysis was performed, that considered a more optimistic scenario in which patients with a history of BE that were cured after RFA-EMR were thought to present a lower rate of disease progression than NDBE patients. Probabilistic sensitivity analyses (PSA) were performed using a Monte Carlo simulation in which distributions for specific model input variables (gamma for costs and utility values, beta for eradication, stricture and perforation rates and triangular for transition probabilities) were assigned and 1,000 iterations were performed. The results of the sensitivity analyses were stratified by thresholds for the dominance and cost-effectiveness within the commonly accepted willingness-to-pay (WTP) threshold in Spain (€30,000/QALY) 22.
RESULTS
The following results were obtained for the different treatment strategies in the two patient cohorts (HGD and LGD): LYG for each health state; cost of treatment; QALYs gained and ICER; number of esophagectomies, chemoradiotherapy and EAC cases prevented; NNT to prevent one esophagectomy, one chemoradiotherapy treatment, one EAC, or one endoscopic surveillance visit; and costs saved per patient-year via the prevention of esophagectomies, chemoradiotherapy and/or endoscopic surveillance visits.
Base-case results
RFA-EMR in the SoC of BE patients with HGD
This strategy avoided 62.1% of esophagectomies, 62.2% of chemoradiotherapy courses and 8.1% of endoscopic surveillance visits. This resulted in savings of 1,402 per patient-year which was equivalent to €21,023 per patient over the time horizon of the study. After introducing RFA-EMR into the SoC of BE patients with HGD, the NNT in order to prevent one esophagectomy, one chemoradiotherapy course or one endoscopic surveillance visit was 1.61, 27.48 and 2.80, respectively. The cost-effectiveness analysis showed that introducing RFA into the SoC of BE patients with HGD would be a dominant therapeutic strategy due to its lower cost (€-11,430 per patient) and the greater LYG and QALY gained compared with SoC alone (0.67 LYG and 1.23 QALY gained per patient) (Table 3).
Table 3 Results: cost-effectiveness analysis for patients with initial HGD and LDG, respectively

HGD: high-grade dysplasia; LYG: life years gained; QALY: quality-adjusted life year; BE: Barrett's esophagus; NDBE: non-dysplastic Barrett esophagus; LGD: low-grade dysplasia; EAC: esophageal adenocarcinoma; RFA-EMR: radiofrequency ablation plus endoscopic mucosal resection; SoC: standard of care; ICER: incremental cost-effectiveness ratio.
RFA-EMR in the SoC of BE patients with LGD
The introduction of RFA-EMR into the SoC of BE patients with LGD compared with SoC alone would provide an additional 0.10 LYG and 0.56 QALY gained per patient. RFA-EMR reduced the risk of esophagectomy, chemoradiotherapy and EAC by 20.0%, 23.9% and 20.7%, respectively. This resulted in savings of €199.0 per patient-year, equivalent to 2,985 per patient over the time horizon of the study. After adding RFA-EMR to the SoC of BE patients with LGD, the NNT in order to prevent one esophagectomy, one EAC, or one endoscopic surveillance visit was 17.96, 46.43 and 1.20, respectively. Nevertheless, the cost-effectiveness analysis showed that introducing RFA-EMR into the SoC of BE patients with LGD would result in an incremental cost of €8,087 per patient over the time horizon of the study. This resulted in an ICER of €12,865 per QALY gained (Table 3).
Sensitivity analysis
RFA-EMR in the SoC of BE patients with HGD
The OWSA results for the HGD cohort showed that the greatest variations in the ICER resulted from changes in the time horizon of the model, the disutility and the cost associated with esophagectomy together with the cost of RFA. Despite this, the resulting ICER remained dominant in all OWSA scenarios (Fig. 2A). In addition, PSA confirmed these results. After 1,000 simulations, 100% of cases supported the dominance of adopting RFA-EMR into the SoC of HGD patients (Fig. 2B).

Fig. 2 Sensitivity analyses results of introducing RFA-EMR into the SoC of BE patients with HGD. A. One-way deterministic sensitivity analysis. Tornado diagram. B. Probabilistic sensitivity analysis. Incremental cost-effectiveness plane (RFA-EMR: endoscopic treatment based on radiofrequency ablation plus endoscopic mucosal resection; SoC: standard of care; BE: Barrett's esophagus; HGD: high-grade dysplasia; CE-IM: complete eradication of intestinal metaplasia; ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life year).
RFA-EMR in the SoC of BE patients with LGD
The cost-effectiveness of adding RFA-EMR into the SoC of LGD patients would be more favorable (with a lower ICER and greater avoided costs) when a minimum improvement in disease progression after CE-IM was considered. When considering that CE-IM after RFA-EMR in LGD patients would provide a 20% reduction in disease progression compared with the natural course of NDBE; the base case ICER would also decrease from €12,865 to €10,122 per QALY gained and the percentage of esophagectomies, chemoradiotherapy courses or EAC cases avoided would rise. These clinical benefits would lead to an additional cost saving of 889 per patient (a 29.8% relative increase) (data not shown).
OWSA results for the LGD cohort showed that the greatest variations in the ICER resulted from changes in the cost of RFA, the utility score of cured patients with a history of BE, the time horizon of the model and the age at diagnosis. However, none of the resulting scenarios surpassed the commonly-accepted WTP threshold in Spain (Fig. 3A) 22. The PSA confirmed these results. After 1,000 simulations, 100% of cases supported the cost-effectiveness of adopting RFA-EMR into the SoC of LGD patients and remained below the commonly-accepted WTP threshold for adopting new health technologies in Spain 22 (Fig. 3B).

Fig. 3 Sensitivity analyses results of introducing RFA-EMR into the SoC of BE patients with LGD. A. One-way deterministic sensitivity analysis. Tornado diagram. B. Probabilistic sensitivity analysis. Incremental cost-effectiveness plane (RFA-EMR: endoscopic treatment based on radiofrequency ablation plus endoscopic mucosal resection; SoC: standard of care; BE: Barrett's esophagus; LGD: low-grade dysplasia; NDBE: non-dysplastic Barrett esophagus; HGD: high-grade dysplasia; EAC: esophageal adenocarcinoma; ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life year).
DISCUSSION
There is increasing debate regarding the use of resources to implement preventive strategies to reduce the rising costs of healthcare associated with EAC, one of the fastest growing cancers in the last three decades 23) (24. In this regard, economic analysis can be useful for the quantitative estimation of the resources needed to implement competing clinical management strategies for BE patients under uncertain conditions. This study is the first economic evaluation of RFA (RFA-EMR in Spain) for the treatment of different stages of BE according to the clinical practice in Spain.
There is a general agreement with regard to the efficiency of RFA for the management of HGD patients 5) (10) (25. In this base-case analysis, RFA-EMR dominated over SoC alone in these patients. These results are in line with other cost-effectiveness analyses that concluded that RFA-EMR was a valid alternative treatment choice for HGD 26) (27) (28) (29) (30.
To date, the subgroup of patients with LGD is the main area of controversy with regard to the ablative treatment of BE 23. However, recent evidence has confirmed the long-term efficacy and safety of RFA for the treatment of LGD 14) (31) (32 and this has been acknowledged by health technology evaluation agencies 3. The analysis presented here used a different pattern of clinical practice but was similar to studies carried out in the USA 27) (29 and Europe 30) (33. These studies have shown that RFA-EMR with SoC was a cost-effective strategy compared to SoC alone in BE patients with LGD.
Some authors suggest that ablation might be preferable in patients with LGD if endoscopic surveillance could be reduced or even discontinued after a successful eradication 23) (27) (29. In our analysis, RFA-EMR remained cost-effective even when endoscopic surveillance was maintained after CE-IM and, overall, reduced the total number of surveillance sessions required due to a lower probability of progression to health states that require a more intensive follow-up. As further post-ablation data becomes available, it is likely that there will be more discussion of the optimal management strategy.
The analysis presented here has some limitations. Due to the current lack of Spanish guidelines for the diagnosis and treatment of patients with BE, it was estimated that the management of BE in Spain would not differ significantly from the European recommendations 3) (11) (12) (25. Consequently, in order to ensure that the SoC considered was representative of Spanish clinical practice, a panel of clinical experts was consulted in order to provide and validate the main use of resources and the current SoC for HGD and LGD patients. Recent international guidelines have started to recommend endoscopic ablative therapy not only for BE patients with HGD, but also for LGD 3) (6) (7) (12) (34. However in Spain, ablative treatment is still only available in a few specialized centers and surgery remains the most common therapeutic choice when patients with BE develop non-nodular HGD. Nevertheless, other valid treatment alternatives, such as endoscopic submucosal dissection should be further evaluated as their use becomes more frequent in clinical practice. On the other hand, concomitant treatment alternatives such as proton pump inhibitors 35),(36 were not considered in this analysis as they implied a limited cost that was equal for all interventions compared in this study. Moreover, the analysis was based on a mathematical model and therefore has limitations due to the variability in the data sources included in the model and the assumptions applied in their absence. However, these are inherent issues of disease modeling and extensive sensitivity analyses were performed.
The additional LYG in the EAC health state when newly diagnosed HGD patients are intervened with RFA-EMR instead of esophagectomy, favors esophagectomy and might indicate that the use of RFA-EMR would lead to a longer period of time with cancer. In fact, our results derive from the assumption that patients that underwent esophagectomy fully recovered from BE and had no risk of progression to cancer or mortality due to the esophagectomy or relapsed cancer. This does not occur in RFA-EMR treated patients, these patients remain at risk of progression to cancer and of undergoing an esophagectomy (with an associated risk of mortality). Future research should consider study the efficacy of RFA according to the length of BE, as this could help identify patients with a higher risk of neoplastic progression in whom endoscopic ablation may be more beneficial. This information could aid the modeling of the cost-effectiveness of RFA in NDBE patients, where RFA efficacy is still unknown 2) (3.
Although ablative therapy followed by endoscopic surveillance has been reported to reduce the recurrence of IM 1) (15, it is not uncontroversial 30. Consequently, the conservative assumption was made that the relapse rates of cured patients with a history of BE after RFA-EMR and the disease progression rates of NDBE patients would be the same. This assumption also balances the decision not to include the possibility of buried crypts after RFA or the possibility of a misdiagnosis. However, more optimistic scenarios were considered in the structural sensitivity analysis and these showed that the base case clinical and economic results could improve when a minimal durability effect was considered after ablative therapy.
Critical parameters have been identified and these should guide further research. According to the sensitivity analysis results, the difference in terms of utilities between non-EAC vs. EAC health states was one of the main drivers of the cost-effectiveness analysis. Together with the lack of specific utilities for Spanish BE patients, this may be considered as an additional study limitation. Further research on the quality of life of patients with BE would add value to these findings.
Overall, our analysis indicates that the introduction of RFA-EMR into the SoC of BE patients diagnosed with HGD or LGD results in clinical benefits. On the one hand, it would translate into additional LYG and QALYs and on the other hand would reduce the use of high-cost invasive treatment alternatives for advanced BE. According to the estimated NNT, one esophagectomy, which is a highly-invasive procedure associated with a risk of mortality, could be avoided by treating 1.61 patients diagnosed with BE and HGD or 17.96 patients with BE and LGD with an initial RFA-EMR.
Finally, from a healthcare decision-maker perspective, when a WTP threshold of €30,000/QALY gained in the Spanish setting is considered, the addition of RFA-EMR to the SoC would be the intervention of choice for the management of BE patients with HGD or LGD in the clinical practice in Spain.














