My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 n.3 Madrid Mar. 2018
https://dx.doi.org/10.17235/reed.2018.5169/2017
ORIGINAL PAPERS
Inter and intra-observer concordance for the diagnosis of portal hypertension gastropathy
1Servicio de Aparato Digestivo. Corporació Sanitària Universitària Parc Taulí. Universitat Autònoma de Barcelona. Barcelona, España
INTRODUCTION
Portal hypertension gastropathy (PHG) is a lesion of the gastric mucosa in patients with portal hypertension (PHT). It is characterized by the presence of dilatation and ectasia of the capillaries and venules of the mucosa and gastric submucosa at the level of the gastric fundus, in the absence of any inflammatory phenomena. These lesions may occur in the form of a mosaic pattern, including erythema, red spots or diffuse hemorrhage of the gastric mucosa. PHG was first described by McCormack et al. 1 in 1985. Subsequently in 1988, Thiruvengadam described PHT enteropathy (PHE) after observing duodenal lesions similar to PHG during endoscopies of three patients with cirrhosis and acute or chronic blood loss 2.
The prevalence of PHG varies between 7% and 98% 3) (4) (5) (6) (7 according to published series. There are a number of reasons for the wide disparity in these results: a) differences with regard to patient selection; b) the lack of standardized diagnostic criteria; c) the use of different classifications of PHG grade; and d) the existence of inter and intra-observer differences. With regard to PHE, the few studies in the literature report a prevalence between 15% and 96.8% in patients with cirrhosis and PHT. However, the number of patients with PHE is probably much higher, with figures between 40% and 96.8% in some series 8) (9) (10) (11) (12) (13) (14) (15. As in the case of PHG, these discrepancies are probably due to differences in the criteria used for patient selection and endoscopic interpretations.
The diagnosis of PHG is made by upper digestive endoscopy. The most frequent location is the fundus, followed by the gastric body and antrum. Although the lesions may be present in any part of the digestive tract. Currently, three classifications are used for diagnosis, based on the endoscopic appearance of the mucosa:
McCormack's classification 1, which divides gastropathy into two forms (mild and severe) based on the shape and extent of redness or the presence of hemorrhage.
Tanoue's classification 16 (1992), of three grades (mild, moderate and severe). The endoscopic description is more detailed but has a lower specificity and reproducibility and greater inter and intra-observer differences 17.
The New Italian Endoscopy Club (NIEC) 18, a much more complex and subjective system, which is less frequently used.
Very few studies in the literature have evaluated inter and intra-observer concordance in portal gastropathy with regard to the different classifications. The aim of our study was to assess the degree of inter and intra-observer agreement regarding the presence of gastro-enteropathy in patients with liver cirrhosis.
MATERIAL AND METHODS
Participants
All patients with liver cirrhosis with an indication for an upper gastrointestinal endoscopy were included. Patients were recruited from the outpatient clinic, hepatology day hospital or hospitalization unit at our center between May 2009 and April 2013. The patients met all the inclusion criteria and none of the exclusion criteria.
The inclusion criteria were: age 18 or over and liver cirrhosis diagnosed by anatomopathological criteria (fibrosis and regenerative nodules) 19, radiological criteria (heterogeneous liver, nodular surface, atrophy of the right hepatic lobe, hypertrophy of the caudate lobe, splenomegaly, presence of collateral circulation) 20) (21, clinical criteria (presence of hepatic encephalopathy, ascites or varices in gastroscopy) or laboratory tests (hypoalbuminemia, prolongation of prothrombin time, thrombocytopenia), regardless of the etiology. All patients agreed to participate and provided informed consent after an explanation of the objectives and procedures of the study.
The exclusion criteria were: contraindication for performing a digestive endoscopy; patient's refusal to undergo endoscopy; patients with active upper gastrointestinal bleeding; presence of concomitant diseases with a life expectancy less than one year (hepatocarcinoma stage B, C or D of the Barcelona Clinic Liver Cancer classification, other active neoplasms, etc.).
Patients who agreed to participate were informed that they would undergo enteroscopy rather than a conventional gastroscopy in order to visualize most of the upper digestive tract under sedation.
The study was approved by the Ethics Committee of our center.
Variables of interest
Demographic and clinical data and information regarding liver cirrhosis were collected. During the enteroscopy procedure, the presence of PHG in the fundus, antrum, duodenum and jejunum was assessed prospectively. Enteropathy due to portal hypertension was defined as the presence of erythema, red spots, angiomas and the existence of bleeding. The degree of PHG was measured by the Tanoue classification as it is most commonly used in our clinical practice 16. The presence of other endoscopic lesions such as esophageal varices, erosions and ulcers, among others, was also recorded.
Endoscopic procedure
The enteroscopy was performed under sedo-analgesia, with fentanyl (50 µg) and midazolam or propofol adjusted to the patient's weight in all cases. An Olympus EVIS EXERA II PCF-Q180AL endoscope (pediatric colonoscope) was used to explore the entire duodenum and proximal jejunum in all patients. All enteroscopies were performed by the same endoscopist. During the examination, biopsies were taken or endoscopic treatment was performed based on the endoscopic findings when appropriate. All examinations were performed with the aid of a nurse and an assistant nurse. The endoscopy images were videotaped for subsequent evaluation of the results.
Enteroscopy evaluation
Three endoscopists with more than ten years of experience (including the endoscopist who had performed the exploration) independently evaluated all the endoscopy images. They were all blind to the clinical data of the patients, the enteroscopy report issued and the evaluations of the other observers. In each case, the location of the lesions (fundus, antrum, duodenum or jejunum) and the severity were recorded in accordance with the classifications of McCormack (non-gastropathy, mild, severe) 1 and Tanoue (non-gastropathy, mild, moderate, severe) 16.
Prior to the evaluation of the lesions, a consensus meeting was held and the images corresponding to the two PHG classifications (McCormack and Tanoue) were visualized. In the case of a doubt during the viewing of the videos, a document with the images was available for clarification purposes (Fig. 1).

Fig. 1 Example of endoscopic classifications. A. Tanoue's classification. B. McCormack's classification.
Intra-observer concordance was evaluated using the initial assessment (as reflected in the enteroscopy report) and the assessment performed later after viewing the endoscopy video images (in this case only the Tanoue classification was evaluated and the evaluation was carried out by the endoscopist who had performed the test). For the inter-observer correlation, the videotaped endoscopic evaluations were compared by the three endoscopists, using the McCormack and Tanoue classifications.
Statistical methods
The agreement between the assessments of the different endoscopists was measured using contingency tables and Cohen's kappa index. A kappa value greater than 0.4 was considered as acceptable and a value greater than 0.75 was deemed as excellent. Finally, a measure of global agreement was calculated between the measurements obtained by the three observers.
RESULTS
Seventy-four patients who underwent enteroscopy with adequate images were included in the evaluation of the agreement between the endoscopists. The most relevant demographic, clinical and analytical characteristics are presented in table 1. There was a predominance of male patients (71.6%) with an average age of 63.2 years. The most frequent treatments are also shown in table 1. Of the 18 patients with a previous gastroscopy, four (22.2%) had a history of PHG in a previous endoscopy and PHG was not observed in the endoscopy performed in this study in two cases (50%). Seventeen patients had food debris in the stomach which made it difficult to visualize the digestive mucosa.
Table 1 General characteristics

SD: standard deviation; CHC: chronic hepatitis C; PHT: portal hypertension.
Gastropathy was found in 26 (37%) patients which was mild in 15, moderate in eight and severe in three cases. Finally, a concordance study of the enteropathy was not performed as compatible lesions were identified in only one patient.
Results of the concordance study
Intra-observer concordance
The concordance between the initial endoscopic assessment and the subsequent assessment of the video-recorded images for the presence or absence of gastropathy was low, with a kappa value of 0.12. The agreement regarding the degree of PHG was also low, with a kappa value of 0.22. As PHG is located predominantly in the fundus, this site was analyzed separately. In addition, the agreement of the degree of PHG between the initial and the deferred observations was low, with a kappa value of 0.22.
Inter-observer agreement
Table 2 shows the correlation between the different observers for the evaluation of the presence or absence of PHG according to the Tanoue and the McCormack classifications. The greatest differences were due to disagreements in interpretation, specifically whether PHG was present, though mild, or absent.
The agreement between the different observers regarding the severity of the PHG that was jointly assessed in the fundus and the antrum was very low (Table 3). The overall measure of agreement between the three observers was calculated using the Tanoue and McCormack classifications. The kappa values obtained were 0.16 and 0.27, respectively, indicating the absence of concordance.
DISCUSSION
Our study shows that both intra and inter-observer agreement regarding the assessment of portal gastropathy are very low, both in terms of the presence or absence of PHG and the degree.
In 1997, Carpinelli et al. analyzed the degree of concordance of 32 different endoscopists assessing PHG using the classification of the New Italian Endoscopy Club. The recorded gastroscopy images of 2,720 patients were reviewed, 721 of whom had PHG (566 cirrhotic). The degree of concordance was greater when PHG was classified as mild or severe than when classified as an intermediate stage. As a result of this study, it was recommended that PHG should be defined in only two stages, mild and severe, using the McCormack classification 22. Another study included six expert endoscopists who reviewed the images of 120 patients with PHG and 22 controls with gastritis and grouped the lesions according to McCormack's two-grade and Tanoue's three-grade classifications. The concordance and reproducibility were significantly higher in the two-degree classification 17.
Only one study to date has performed an intra-observer assessment. Yoo et al. 17 compared the degree of intra-observer concordance between the McCormack and Tanoue classifications and observed a low degree of agreement in the Tanoue classification (kappa = 0.43) and a moderate agreement in the McCormack classification (kappa = 0.63). The degree of agreement was higher than that observed in our study. Yoo et al. suggested that McCormack's classification of PHG severity, which only has two categories, would be easier to reproduce 22.
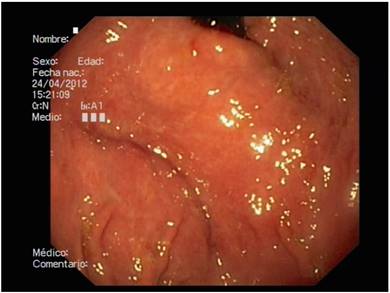
In our study, the degree of inter-observer agreement was very low for both the presence and the degree of PHG, using both classifications. We found that the degree of agreement was greater between two of the three endoscopists. This was due to the fact that one endoscopist classified a subgroup of patients with slight changes as "normal", while the other two defined this as PHG. This result highlights the difficulty of classifying the "minimal changes" in the mucosa which are often observed in patients with liver cirrhosis (Fig. 2). The endoscopist that reported the discordant data had more than ten years of experience in upper endoscopy and there were no significant differences with respect to the two other colleagues.
Our data differ from those reported in the study by Yoo et al. 17. This study found a moderate degree of inter-observer concordance in both classifications (though lower in the three-degree system, kappa 0.44 vs kappa 0.52). Several authors 17) (22) (23 have suggested that the lack of agreement may also be due, in part, to the complexity of the description of the endoscopic findings. Thus, Tanoue's three-degree classification seems to have more limitations in terms of reproducibility and greater intra and inter-observer differences. However, this difference was less evident in this study, probably due to the fact that a very low concordance was obtained with both classifications.
Our results suggest that neither classification offers satisfactory reproducibility in clinical practice, even though they are habitually used and recognized in the Baveno consensus 24. Of the two methods, the McCormack classification may have a higher reproducibility.
This study is relevant as it shows that there is no reliable endoscopic classification for this pathology. The different classifications have a high discordance between observers, so it is not possible to objectively evaluate their evolution in a given patient, especially in patients with clinically significant PHG suffering from anemia.
One possible reason for the disagreement regarding the presence or absence of PHG is the fact that the endoscopist knows that the patient has cirrhosis. This may generate a bias, with a tendency to classify minimum changes as mild PHG, which would be classified as nonspecific in another patient. This carries the risk of attributing the presence of anemia in these types of cases to the PHG and thus neglecting other possible causes. A limitation of the study was that only the Tanoue classification was used in the initial endoscopy, so the intra-observer correlation of the McCormack classification could not be evaluated.
This study questions the utility of the different classifications for PHG. It is important to confirm our data in a study with a larger patient cohort with all three endoscopic classifications, both at the time of the initial examination and during the subsequent review.
In conclusion, the McCormack and Tanoue classifications have a very low degree of intra and inter-observer agreement, both for the diagnosis of PHG and the classification of severity. On the basis of our results we cannot recommend either classification for use in routine clinical practice.
BIBLIOGRAFÍA
1. McCormack TT, Sims J, Eyre-Brook I, et al. Gastric lesions in portal hypertension: Inflammatory gastritis or congestive gastropathy? Gut 1985;26(11): 1226-32. DOI: 10.1136/gut.26.11.1226 [ Links ]
2. Thiruvengadam R, Gostout CJ. Congestive gastroenteropathy - An extension of nonvariceal upper gastrointestinal bleeding in portal hypertension. Gastrointest Endosc 1989;35(6):504-7. DOI: 10.1016/S0016-5107(89)72898-4 [ Links ]
3. Primignani M, Carpinelli L, Preatoni P, et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC). Gastroenterology 2000;119(1):181-7. DOI: 10.1053/gast.2000.8555 [ Links ]
4. Sarin SK, Sreenivas DV, Lahoti D, et al. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology 1992;102(3):994-9. DOI: 10.1016/0016-5085(92)90188-5 [ Links ]
5. D'Amico G, Montalbano L, Traina M, et al. Natural history of congestive gastropathy in cirrhosis. The Liver Study Group of V. Cervello Hospital. Gastroenterology 1990;99(6):1558-64. DOI: 10.1016/0016-5085(90)90458-D [ Links ]
6. Iwao T, Toyonaga A, Sumino M, et al. Portal hypertensive gastropathy in patients with cirrhosis. Gastroenterology 1992;102(6):2060-5. DOI: 10.1016/0016-5085(92)90332-S [ Links ]
7. De Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis 2001;5(3):645-63. DOI: 10.1016/S1089-3261(05)70186-0 [ Links ]
8. Vigneri S, Termini R, Piraino A, et al. The duodenum in liver cirrhosis: Endoscopic, morphological and clinical findings. Endoscopy 1991;23(4):210-2. DOI: 10.1055/s-2007-1010658 [ Links ]
9. Boron-Kaczmarzka A, Hryniewicz A, Kemona A, et al. Morphologic changes of small intestine epithelium in the course of post-alcoholic liver cirrhosis. Drug Alcohol Depend 1990;25(3):299-303. DOI: 10.1016/0376-8716(90)90155-8 [ Links ]
10. Menchén L, Ripoll C, Marín-Jiménez I, et al. Prevalence of portal hypertensive duodenopathy in cirrhosis: Clinical and haemodynamic features. Eur J Gastroenterol Hepatol 2006;18(6):649-53. DOI: 10.1097/00042737-200606000-00012 [ Links ]
11. Figueiredo P, Almeida N, Lérias C, et al. Effect of portal hypertension in the small bowel: An endoscopic approach. Dig Dis Sci 2008;53(8):2144-50. DOI: 10.1007/s10620-007-0111-z [ Links ]
12. De Palma GD, Rega M, Masone S, et al. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: A capsule endoscopy study. Gastrointest Endosc 2005;62(4):529-34. DOI: 10.1016/S0016-5107(05)01588-9 [ Links ]
13. Desai N, Desai D, Pethe V, et al. Portal hypertensive jejunopathy: A case control study. Indian J Gastroenterol 2004;23(3):99-101. [ Links ]
14. Gupta R, Saraswat VA, Kumar M, et al. Frequency and factors influencing portal hypertensive gastropathy and duodenopathy in cirrhotic portal hypertension. J Gastroenterol Hepatol 1996;11(8):728-33. DOI: 10.1111/j.1440-1746.1996.tb00322.x [ Links ]
15. Egea J, Fernández T, García AV, et al. Diagnostic and therapeutic features of small bowel involvement in portal hypertension. Rev Esp Enferm Dig 2017;109(12):856-62. DOI: 10.17235/reed.2017.4596/2016 [ Links ]
16. Tanoue K, Hashizume M, Wada H, et al. Effects of endoscopic injection sclerotherapy on portal hypertensive gastropathy: A prospective study. Gastrointest Endosc 1992;38(5):582-5. DOI: 10.1016/S0016-5107(92) 70522-7 [ Links ]
17. Yoo HY, Eustace JA, Verma S, et al. Accuracy and reliability of the endoscopic classification of portal hypertensive gastropathy. Gastrointest Endosc 2002;56(5):675-80. DOI: 10.1016/S0016-5107(02)70116-8 [ Links ]
18. Spina GP, Arcidiacono R, Bosch J, et al. Gastric endoscopic features in portal hypertension: Final report of a consensus conference, Milan, Italy, September 19, 1992. J Hepatol 1994;21(3):461-7. DOI: 10.1016/S0168-8278(05)80329-0 [ Links ]
19. Anthony PP, Ishak KG, Nayak NC, et al. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol 1978;31(5):395-414. DOI: 10.1136/jcp.31.5.395 [ Links ]
20. Hess CF, Schmiedl U, Koelbel G, et al. Diagnosis of liver cirrhosis with US: Receiver-operating characteristic analysis of multidimensional caudate lobe indexes. Radiology 1989;171(2):349-51. DOI: 10.1148/radiology.171.2.2649915 [ Links ]
21. Di Lelio A, Cestari C, Lomazzi A, et al. Cirrhosis: Diagnosis with sonographic study of the liver surface. Radiology 1989;172(2):389-92. DOI: 10.1148/radiology.172.2.2526349 [ Links ]
22. Carpinelli L, Primignani M, Preatoni P, et al. Portal hypertensive gastropathy: Reproducibility of a classification, prevalence of elementary lesions, sensitivity and specificity in the diagnosis of cirrhosis of the liver. A NIEC multicentre study. New Italian Endoscopic Club. Ital J Gastroenterol Hepatol 1997;29(6):533-40. [ Links ]
23. De Macedo GF, Ferreira FG, Ribeiro MA, et al. Reliability in endoscopic diagnosis of portal hypertensive gastropathy. World J Gastrointest Endosc 2013;5(7):323-31. DOI: 10.4253/wjge.v5.i7.323 [ Links ]
24. De Franchis R. Updating consensus in portal hypertension: Report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol 2000;33(5):846-52. DOI: 10.1016/S0168-8278(00)80320-7 [ Links ]
Received: July 20, 2017; Accepted: November 17, 2017











 text in
text in