INTRODUCTION
Gastrointestinal stromal tumors (GIST), although rare, are the most common sub-group of neoplasms that originate from the mesenchymal cells of the gastrointestinal (GI) tract. GIST account for around 1% of all GI tumors 1,2. Epidemiological data show an incidence of GIST of 4.1-21.4 cases per million people per year 1,3,4.
Before the recent advances in immunohistochemical markers, GIST were commonly misdiagnosed as neoplasms originating from muscle cells or the nerve sheath 5. The advances in biomarker determination and the identification of the KIT oncogene mutation (which is seen in around 95% of GIST) have made diagnosis and differentiation easier. This has led to a better appreciation of GIST characteristics 4,6. The clinical presentation of GIST is accepted as nonspecific. Symptoms such as bloating, GI bleeding and abdominal pain may be present if the tumor has grown. However, many cases (especially smaller GIST) are found incidentally during procedures such as endoscopy and computed tomography (CT) 1.
Today, the diagnosis and evaluation of GIST are performed by a pathological examination of the tissue obtained during surgery and contrast-enhanced imaging studies 7. Tumor size, mitotic count and tumor location are currently accepted as factors that determine tumor prognosis 8,9. A complete surgical excision is the only curative treatment for GIST 10,11. However, after its approval by the FDA in 2002, imatinib has been used in KIT-positive GIST and has shown spectacular results 12,13.
As GIST are relatively rare tumors, the majority of data in this field is derived from case reports and case series with low to moderate numbers of cases as well as single and multicenter studies. There are single and multicenter studies on GIST from Turkey 14,15,16. However, the real incidence and prevalence of GIST in Turkey still remain unknown 17. A wide experience is required in order to understand the behavior of the tumor and predict the outcome of the disease. However, this is difficult due to the low frequency of GIST and their infrequent location.
The aim of the study was to convey our 16-year experience of GIST by the evaluation of a broad range of factors, including patient and tumor characteristics, immunohistochemical results, pathological findings, metastasis or recurrence and tumor location with regard to their effects on overall survival.
MATERIAL AND METHODS
Study group
All patients who were diagnosed with GIST in the Department of General Surgery of the Cerrahpaşa Medical Faculty from the Istanbul University during a 16-year period, from January 2000 to December 2015, were retrospectively evaluated. The diagnosis was made by our Pathology Department via a histopathological examination and immunohistochemistry analysis of tissue samples obtained during surgery. All patients included in the study had their tumors surgically resected. Approval was obtained from the local ethical committee. The study was performed according to the principles of the Declaration of Helsinki.
Measurements
A pathological analysis was performed of all tissue samples obtained from GIST patients during surgery by our Pathology Department. Patient follow-up data was obtained from medical files. All relevant patient data, including age, gender, presentation, clinical characteristics, tumor properties and treatment variables, were recorded. The use of imatinib could not be accurately evaluated and was not included in the analyses as some treatment data was lost during the transfer of medical files to a digital platform. The loss of data was limited to drug use and other data of patients were not affected. Tissue samples were processed by the following routine method. Specimens were fixed in 10% formaldehyde, embedded in paraffin, and 5 μm sections were stained with hematoxylin and eosin (H&E). CD117 (c-kit), CD34, smooth muscle actin (SMA), S100 and desmin expression were assessed using specific antibodies. Samples were prepared using the following method. Sections were de-paraffinized and dehydrated (with xylene for ten minutes and alcohol for five minutes), and antigen retrieval was performed via trypsinization (for desmin) or using a pressure cooker (for the other proteins). Sections were incubated with 100 μl of casein for five minutes in order to prevent non-specific antibody binding. Samples were then incubated for 60 minutes with a primary antibody solution (diluted according to manufacturers' recommendations) and incubated with the secondary antibody (in a solution of 1:100 3,3'-diaminobenzidine) for 30 minutes according to manufacturers' recommendations. Finally, the sections were counter-stained with Meyers hematoxylin for one minute, followed by dehydration in alcohol and a xylene wash before mounting. All samples were evaluated by an experienced pathologist in the field. All results were recorded in patient files.
Diagnosis
Currently, the criteria for GIST diagnosis is based on two factors: a) the presence of spindle, epithelioid or mesenchymal tumor cells on histopathological examination; and b) CD117 expression with or without CD34 expression via immunohistochemical staining. All patients were diagnosed according to these two criteria.
Risk and data stratification
The Armed Forces Institute of Pathology (AFIP) criteria (also known as Miettinen risk score) were used for patient risk stratification 18. Tumor size, mitotic rate and tumor location are three factors that determine patient risk group. The groups are very low-risk, low-risk, intermediate-risk and high-risk. Mitotic activity was assessed by counting the number of cells undergoing mitosis under x 50 high-power fields (HPF).
Statistical analysis
All analyses were performed using SPSS v21. Continuous variables are expressed as mean ± standard deviation for normally distributed data and median (minimum-maximum) for non-normally distributed data. Categorical variables are expressed as frequencies (percentage). Survival analyses were performed using the Kaplan-Meier method. Survival time comparisons between groups were performed using the log-rank test. Pairwise comparisons were performed with the Bonferroni correction method. The effects of continuous variables on survival times were evaluated by Cox-regression analysis with a backward conditional method. p values ≤ 0.05 were considered as statistically significant.
RESULTS
One hundred and thirty-five patients (76 males and 59 females) were included in the study, the mean age was 62.8 ± 13.3 years. Sixty-eight (50.4%) patients had tumors located in the stomach, 38 (28.1%) in the small intestine, 18 (13.3%) in the colorectal region and eleven (8.1%) at other sites. Ninety (66.7%) patients had a spindle type tumor, 33 (24.4%) patients had mixed type and 12 (8.9%) patients had epithelioid type tumors. The most common clinical symptom was abdominal pain (65.2%). With regard to Miettinen risk scores, 19 (14.1%) patients had a very low score, 21 (15.6%) patients had a low score, 27 patients had a (20.0%) moderate score and 68 (50.4%) patients had a high score. Eight (5.9%) patients had recurrence, nine (6.7%) patients had metastasis and two (1.5%) patients had lymph node involvement (Table 1).
Table 1 Patient demographics and tumor characteristics (n = 135)

Data shown as a frequency (percentage) and as the median (range). HPF: high-power field.
Mean overall survival time was 121.33 ± 7.00 months and the five-year survival rate was 66.6 ± 4.2%. Overall survival of the patient cohort is shown in Figure 1. Patients with colorectal tumors had significantly lower survival times than patients with tumors located in the stomach (p = 0.001) and small intestine (p = 0.033). Survival times according to tumor location is shown in Figure 2. When all risk groups were compared, survival times were significantly different among the groups (p = 0.001). However, only the moderate risk group and high risk group showed significant differences (p = 0.003) in terms of survival according to the pairwise comparison. There were no significant differences between very-low, low and high risk scores (Table 2). The survival times of patients according to risk groups are shown in Figure 3. In addition, patients with recurrence had a significantly lower survival time (p < 0.001) (Table 2).
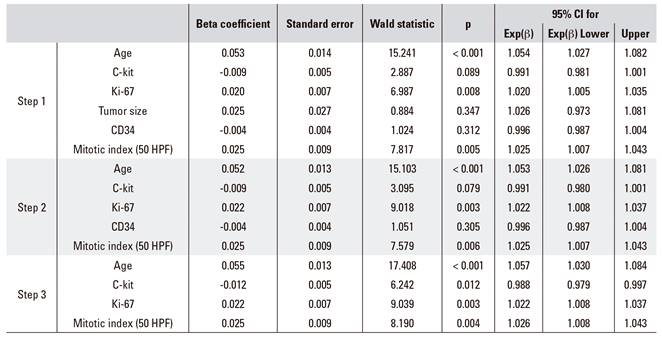
A Cox regression analysis was performed with the continuous variables of age, C-kit, Ki-67, tumor size, CD34 and mitotic index. Age, Ki-67 and mitotic index were poor prognostic factors and C-kit was a good prognostic factor. An increase of one unit in age increased mortality risk by a coefficient of 1.057, an increase of one unit in Ki-67 increased mortality risk by 1.022, and a one unit increase of mitotic index increased mortality risk by 1.026. Furthermore, a one unit increase in C-kit reduced mortality risk by a coefficient of 0.988 (Table 3).
Table 2 Kaplan-Meier and log rank test results for categorical variables

The same letter (a or b) denotes the lack of statistically significant differences between groups.
DISCUSSION
The present study retrospectively evaluated the prognostic factors and survival of a patient cohort diagnosed with gastrointestinal stromal tumors that underwent surgery in a Turkish clinic over a 16-year period. Patient survival was associated with GIST Miettinen risk group, tumor location and recurrence. Furthermore, Ki-67 and mitotic indices were identified as poor prognosis factors while C-kit was identified as a good prognosis factor. These results are by and large in agreement with previous reports, with the exception of tumor size and gender, which were not associated with survival in the present study. The evaluation of factors that affect GIST prognosis is a highly relevant subject and previous single and multicenter studies have identified relationships between survival and various factors.
The age distribution of GIST cases is between 60-65 years of age. However, GIST have been reported in almost all age groups, whereas the gender distribution is the same 4. Cox regression analysis revealed a significant relationship between age and survival time in this study. Various studies have also reported that younger patients have a better prognosis 19,20,21,22. However, younger age was found to be significantly related with higher-risk tumors in a study by Dogusoy et al. 14.
In the present study, gender was not significantly related with survival. A study performed of 46 GIST patients reported a higher five-year survival rate among female cases (52% vs 75%) 21. A cohort study comprised of 1,215 GIST patients also found better survival rates in females compared to males (HR = 1.5, 95% CI = 1.2-1.8) 23. Likewise, a study by Tran et al. found that females were likely to have a lower five-year mortality risk (HR = 0.83, 95% CI = 0.71-0.97). However, two studies by Seker et al. 15 and Bertin et al. 24 reported no significant association between survival and age or gender.
Mitotic index (MI) is a reliable indicator of tumor aggressiveness as a higher MI is suggestive of malignancy 5,25. We found that increased mitotic index significantly increased mortality risk. Various studies have associated a shorter survival with high MI 24,26,27,28. However, one study reported no relationship between MI and prognosis 15.
We found no relationship between tumor size and prognosis. Some studies have reported that large tumors were indicative of poor prognosis 21,26,27,28. These studies had a relatively higher ratio of patients with metastasis and/or higher risk patients compared to our study (6.7% and 50%, respectively). Thus, caution should be taken in the interpretation of our data when patients with metastasis are considered. However, tumor size may not be indicative of prognosis in patients without metastasis.
Currently, the anatomic location of the tumor is accepted as a prognostic factor in the NCCN guidelines 29. A very informative systematic review by Søreide et al. 4, which assessed the data from population-based studies, found that GIST are most frequently found in the stomach (55.6%). Other locations in order to reduce frequency include the small bowel (31.8%), colorectal (6%), various locations (5.5%) and the esophagus (0.7%). Tumor locations in this study included the stomach (50.4%), small intestine (28.1%), colorectal (13.3%) and other locations (8.1%). In our study, Kaplan-Meier and log-rank analyses found that colorectal tumors had significantly lower survival times than tumors located in the stomach (p = 0.001) and small intestine (p = 0.033). While various studies report that a gastric location is suggestive of a better prognosis, there are also studies that report no relationship 15,24. A study that compared the survival of gastric, colorectal and small intestine GIST found that gastric location was suggestive of a better prognosis 20. In a population-based study, gastric location was again found to be highly suggestive of a better prognosis compared to an intestinal location (mortality risk of intestinal vs gastric: HR = 1.609) 19. Various other studies also report that gastric location is indicative of a better prognosis 20,30,31. Our results were in agreement with the current literature, except that we found no significant difference between the survival times of gastric and intestinal tumors.
Survival based on the presence or absence of metastasis was performed due to the low number of patients with metastasis in our study. The presence of metastasis was not related to survival. However, this may also be due to the low number of cases with metastasis in our study and the reduced statistical power. A study by Seker et al. 15 found that patients that underwent a R0 resection had a significantly higher 5-year overall survival rate (75.7% vs 79.7%) than patients with metastasis, and also a higher median overall survival.
In the present study, patients with tumor recurrence had a significantly shorter survival. As previously stated, resection of the tumor is currently the only curative option for GIST. Tumor size plays an important role in recurrence rate; larger tumors have a drastically increased chance of recurrence (50%), even after a wide resection 30,32. However, the introduction of imatinib into the clinic in 2002 has reduced metastasis risk and recurrence rates 20.
The literature findings conclude that GIST may express desmin (2-13.4%), smooth muscle actin (30-40%) and S-100 (5-10%) with varying degrees of association with prognosis 24,33,34. In our study, the positive expression rates were 8.8% for desmin, 31.8% for smooth muscle actin and 5.1% for S100, which is in agreement with the literature. Various studies report that desmin and SMA are positively correlated with a better prognosis 14,21,35,36. However, a study by Bertin et al. 24 reported that high SMA positivity was associated with shorter survival. With regard to S100 expression, Miettinen et al. 35,36 reported that S100 expression is indicative of malignancy and is more frequent in small intestinal GIST. They suggested that S100 may be a poor prognosis indicator for a gastric location but not for a small intestine location. However, the low number of cases in the study is a limiting factor. Two studies have reported no association between prognosis and S100 positivity 14,24. In the current study, no relationship was found with these parameters and survival.
Ki-67 is an accepted accurate marker for cell proliferation and is correlated with mitotic activity. The Ki-67 index is usually reported as a percentage and various studies state that a value higher than 10% is indicative of poor prognosis of GIST 21,26,37. Cox regression analysis revealed that Ki-67 significantly increased mortality risk. This is in agreement with previous reports in the literature. A study conducted by Dogusoy et al. 14 reported that gastric and small intestine GIST had lower Ki-67 index values and that increased values were correlated with increased risk. Furthermore, Pyo et al. 38 found high Ki-67 values were correlated with poor prognosis and a higher risk of recurrence.
The strengths of our study are the following. The association of a large range of variables with prognosis in 135 GIST cases was assessed. The association of these variables with survival was assessed and regression analyses of all continuous variables was performed with backward conditional methods. Various limitations of our study also exist. Firstly, this was a single-center study which may have resulted in limited patient numbers and bias. However, there was a large group of patients considered as diffuse and in line with the literature in terms of their characteristics. However, there were low numbers of cases with metastasis and recurrence. Secondly, relapse-free survival was not assessed due to a low rate of relapse (5.9%). However, the systematic review by Ozer-Stillman et al. 39 reports a very low variation between relapse-free and overall survival in GIST. Thirdly, imatinib use could not be analyzed in our patient cohort due to a loss of data during the transfer of medical records from patient files to the digital platform.
CONCLUSION
Identifying the role of immunohistochemical markers beyond the diagnosis of GIST is a popular topic. This may be due to the fact that if a correlation was found, this would make the estimation of prognosis much easier. However, studies have produced conflicting results on this matter and we believe that most of these conflicts arise due to two major reasons: a) older studies which were conducted before the breakthrough of imatinib include a larger number of patients; and b) the heterogeneous distribution of case characteristics. These two factors result in differences in outcomes (such as survival) and patient characteristics (such as the number of relapse and metastasis). Nevertheless, our findings were consistent with most reports in the literature with regard to factors such as age, risk group and recurrence, which were significantly associated with prognosis. However, there are differences between our study and the literature. These differences were noted in detail and explanations were brought forward in the discussion.
Researchers that aim to evaluate survival in GIST may consider re-evaluating factors that were studied during the pre-imatinib era. The use of imatinib may have altered the impact of these factors on prognosis, thus previous assumptions may now be irrelevant. As a final word, in the absence of a national cancer registry, identifying, reporting and evaluating results of rare cancers such as GIST is a cumbersome task. In fact, there may not be sufficient data for population-wide recommendations and interventions. However, we feel that the data presented has some important points and will help with the identification of future directions.