INTRODUCTION
Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease. Ursodeoxycholic acid (UDCA) is the treatment of choice for PBC and studies have shown its efficacy for the improvement of liver biochemistry and a delay in histological progression 1. However, 25-50% of patients treated with UDCA experienced a suboptimal biochemical response. Furthermore, these patients have a significantly worse survival rate compared to those with a good response 2,3,4,5. Therefore, there is an urgent need for a second-line therapy.
Fenofibrate is a peroxisome proliferator-activated receptor-α (PPAR-α) agonist. Early studies have reported the effect of fenofibrate for the reduction of serum ALP and immunoglobulin M levels in PBC patients 6,7. Recent studies have also shown that adding fenofibrate to UDCA could improve clinical symptoms, as well as the biochemical and immunological response, with good safety profiles 8,9,10,11. However, the follow-up duration in most studies was short and the survival benefit was not well documented. In addition, the safety of fenofibrate in cirrhotic patients has not been well addressed.
Therefore, this retrospective study of 39 PBC patients with or without cirrhosis was conducted to clarify the efficacy and safety of long-term (up to 60 months) fenofibrate use in PBC patients with a suboptimal response to UDCA.
PATIENTS AND METHODS
Patients
This retrospective study enrolled PBC patients who received fenofibrate and UDCA combination therapy at the Beijing Friendship Hospital, Capital Medical University, between January 2010 and January 2017. The inclusion criteria of the study were as follows: a) an established diagnosis of PBC based on the American Association for the Study of Liver Diseases (AASLD) guidelines 12; and b) a suboptimal response to UDCA which was defined as ALP levels > 1.5 times the upper normal limit (UNL) after at least one year of treatment with UDCA at 13-15 mg/kg/day 13,14. The exclusion criteria were: a) evidence of concomitant liver disease; b) ALT > 5 × UNL; c) IgG > 2 × UNL; d) moderate or severe interface hepatitis; and e) incomplete data for analysis (missing biochemistry results at key time points).
Cirrhosis was diagnosed histologically (when available) or clinically according to the modified diagnosis criteria from the Korean Association for the Study of the Liver 15 as follows: a) esophageal varices on endoscopy, excluding non-cirrhotic portal hypertension; or b) two of the three following criteria:
Imaging studies (ultrasound, computed tomography [CT] or magnetic resonance imaging [MRI]) showing any signs of cirrhosis such as an irregular liver surface, granular or nodular liver parenchyma, or splenomegaly (spleen thickness > 4.0 cm or > 5 rib units).
Platelet count < 100 × 109/l, without other causes.
Serum albumin < 35.0 g/l or INR > 1.3 or prolonged prothrombin time > 3 seconds.
Decompensated cirrhosis was defined as the development of variceal bleeding, ascites or hepatic encephalopathy.
Methods
All patients received fenofibrate at 200 mg/day in addition to UDCA at 13-15 mg/kg/day. Patients were followed up every three to six months for blood biochemistry tests including ALP, γ-GT, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin and creatinine. Demographic, baseline and follow-up data were obtained from electronic medical records and via telephone interviews. Adverse events were self-reported and documented in the clinical records.
UK-PBC risk score and GLOBE score
The UK-PBC risk score and the GLOBE score were used to predict the estimated probability of liver-related death or the need for liver transplantation. The UK-PBC risk score calculator is available at http://www.uk-pbc.com/resources/tools/risk calculator. The GLOBE score is available at http://www. globalpbc.com/globe.
Evaluation of renal dysfunction
Fenofibrate-related nephrotoxicity was defined as an increase in serum creatinine of 0.3 mg/dl after fenofibrate therapy, without other known causes of nephrotoxicity. This definition is adopted from the Acute Kidney Injury Network 16,17,18. eGFR values were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and the Modification of Diet in Renal Disease (MDRD) equation 19,20.
Transient and severe liver injury
According to the consensus by the international DILI Expert Working Group of clinicians and scientists 21, transient liver injury was defined as an increase in ALT level between 2 UNL and 5 UNL and bilirubin < 2 UNL. Severe liver injury was defined as ALT ≥ 5 UNL or ALP ≥ 2 UNL or ALT ≥ 3 UNL with an associated bilirubin of ≥ 2 UNL.
Statistical analysis
Continuous variables are expressed as means with standard deviations (SD), or the median (interquartile range [IQR]). Categorical variables were summarized by counts and percentage values. The paired Student's t-test or Wilcoxon signed-rank test were used to compare biochemical test data, the UK-PBC risk score and GLOBE score at different time points with those at baseline. Differences in non-continuous variables were analyzed with the Chi-squared test or Fisher's exact test. Significance was offset at p < 0.05 in all statistical tests. The statistical analyses were performed using the SPSS statistics, version 20.
RESULTS
Study population
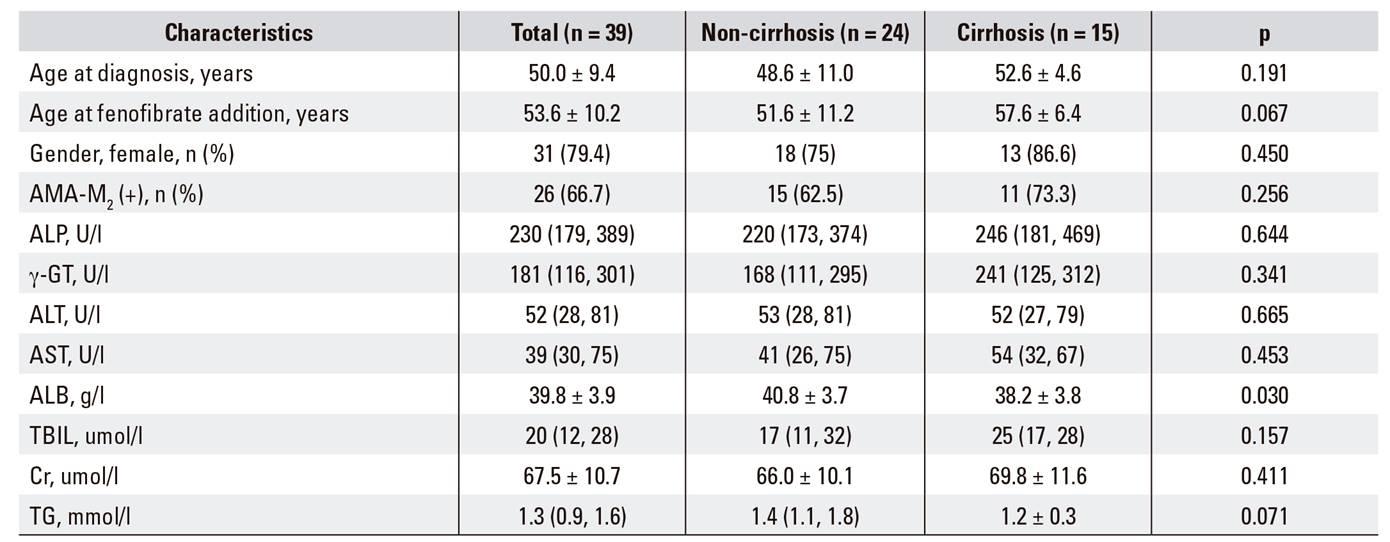
Thirty-nine patients who met the inclusion criteria were given combination therapy for a median duration of 20 months (range: 0.5-60 months). All 39 patients were included in the safety evaluation. Thirteen patients were excluded from the efficacy analysis. Five received fenofibrate for less than one year, two had poor adherence and six withdrew from fenofibrate treatment due to side effects. Figure 1 shows the study flowchart. Only four patients finished fenofibrate therapy at 36 months, two at 48 months and two patients at 60 months. Therefore, the efficacy analysis was focused at 24 months. Among the remaining 26 patients, eleven (42.3%) had cirrhosis, including two patients with ascites and one patient with a history of hepatic encephalopathy at baseline. Demographic and baseline characteristics of the patients are summarized in Table 1.
The efficacy of fenofibrate add-on therapy related to the liver biochemistry analysis
Liver biochemistry at baseline and at three, six, 12 and 24 months of combination therapy are summarized in Figure 2. Serum ALP levels decreased significantly from 215 (185, 326) U/l at baseline to 128 (106, 194) U/l after six months and then to 124 (100, 181) U/l after one year. Serum ALP and -GT reduced significantly as early as three months and persisted until the end of the study. This effect was observed in both the cirrhotic group (n = 11) and the non-cirrhotic group (n = 15). Normalization of ALP was observed in three (27%) and nine (60%) of cirrhotic and non-cirrhotic patients, respectively (p = 0.023). AST, ALT, total bilirubin and albumin levels remained stable during the follow-up.

Fig. 2 Dynamic changes in serum ALP, -GT, serum creatinine and eGFR with fenofibrate add-on therapy for PBC after a suboptimal response to UDCA. Serum ALP and -GT levels were significantly decreased after three, six, 12 and 24 months of add-on therapy in all patients (a and b) and in patients with or without cirrhosis (e and f). Serum creatinine and eGFR worsened after 24 months of add-on therapy in all patients (c, d) and in patients with or without cirrhosis (f and h). All data are shown as a median (IQR), *p < 0.05.
The effect of fenofibrate add-on therapy on UK-PBC risk score and GLOBE score
The predicted three-, five-, and ten-year survival rates by GLOBE score at baseline, 12 months and 24 months of combination therapy were calculated. Compared with baseline, the GLOBE scores were significantly improved at 12 months of combination therapy. However, there was no significant difference at 24 months of treatment (Table 2). The changes in UK-PBC risk scores were not statistically significant either at 12 months or 24 months.
The adverse events and drop-outs
Adverse events are listed in Table 3. The elevation of liver enzymes was the most frequent side effect of fenofibrate. Three patients developed transient liver injury and four patients developed severe liver injury (Fig. 3). The seven patients who developed fenofibrate induced liver injury had significantly higher baseline levels of total serum bilirubin, ALP, γ-GT, ALT, AST and a younger age as compared to those who did not suffer a liver injury. Fenofibrate was discontinued in one patient due to an allergic reaction and due to headaches and insomnia in another case. Other reported symptoms including edema, arthralgia and heartburn were self-limited.

Fig. 3 Liver injury after fenofibrate add-on therapy for PBC with a suboptimal response to UDCA. A and B. Severe liver injury (cases 1-4). Both ALT and total bilirubin dramatically elevated and returned to normal or baseline after discontinuation of fenofibrate. C and D. Transient ALT elevation (cases 5-7). Only the ALT level was slightly elevated after one month of fenofibrate add-on therapy; ALT levels returned to baseline without stopping fenofibrate treatment.
The effects of fenofibrate add-on therapy on serum creatinine levels and eGFRs
In the cirrhotic group, the mean serum creatinine level remained stable at one year of therapy compared to baseline (73.7 ± 15.8 µmol/l versus 72.4 ± 12.8 µmol/l, p = 0.381). However, the levels increased after two years of therapy (80.3 ± 18.6 µmol/l versus 72.4 ± 12.8, p = 0.024). In the non-cirrhotic group, the mean serum creatinine level increased from 68.5 ± 11.2 µmol/l at baseline to 71.4 ± 13.3 µmol/l (p = 0.23) after one year of fenofibrate treatment and then to 74.9 ± 12.5 µmol/l (p = 0.002) after two years of fenofibrate treatment.
The eGFR was calculated using both the CKD-EPI and the MDRD. Since there were no significant differences between the eGFR values calculated with the two equations, only the results for the eGFR calculated by CKD-EPI are shown (Fig. 2). Fenofibrate was associated with a significantly reduced eGFR from baseline to two years.
Fenofibrate-related nephrotoxicity was observed in three patients after one year of fenofibrate therapy. The renal function improved in one patient one month after stopping fenofibrate. Whereas, the eGFR also returned to baseline in two other patients who continued to take fenofibrate.
DISCUSSION
In the present study, we found that two years of fenofibrate add-on therapy could significantly reduce serum ALP and γ-GT levels in non-cirrhotic and cirrhotic patients with a suboptimal response to UDCA. However, this therapy did not improve the UK-PBC Risk Score and GLOBAL score. The elevation of liver enzymes was the most frequent side effect. More importantly, worsening of renal function was observed after two years of fenofibrate therapy.
Our findings are consistent with previous studies of the efficacy of fenofibrate for the improvement of serum ALP levels 22,23. Not surprisingly, we found that the normalization of ALP was observed in 60% of non-cirrhotic patients and 27% of cirrhotic patients. Thus, indicating that cirrhosis is an unfavorable factor for the prediction of the efficacy of fenofibrate. In addition, we have found that the beneficial treatment effect on ALP and -GT appeared as early as three months and persisted after 24 months. This may potentially be used as an early predictor of fenofibrate efficacy. However, we found that two years of fenofibrate add-on therapy did not improve the UK-PBC and GLOBE score which have been recently validated as five-year prognostic predictors in Chinese patients with PBC 24.
Fenofibrate is generally well tolerated. However, patients did experience side effects in our study, and liver enzymes was the most common. Although patients with transient liver injury had a stable level of serum bilirubin, three of four patients with severe liver injury experienced a dramatic increase in serum bilirubin. Fortunately, the liver enzyme and serum bilirubin level returned to baseline after stopping fenofibrate treatment. However, it is noteworthy that a recent study showed an accelerated rise in serum bilirubin with fenofibrate in the setting of advanced PBC 23. It is of note that two of three patients with elevated serum bilirubin had cirrhosis. Although our results showed that total bilirubin was stable during follow-up in the cirrhotic group, we still need to be cautious of serum bilirubin elevation in patients with cirrhosis.
Fenofibrate-associated nephrotoxicity has been reported in non-PBC populations 25,26,27,28. Risk factors of this adverse effect include age, preexisting renal diseases, high-dosage fenofibrate and the concomitant use of angiotensin-converting enzyme-inhibitors or calcium channel blockers 25,29. Interestingly, SCr increases due to fenofibrate were transient and reversible, even without treatment discontinuation 26,27,30. Case reports of PBC patients have shown that creatinine was reversibly increased 10,23. In our study, we found that both serum creatinine and eGFR worsened over time, especially after two years of fenofibrate therapy. However, this finding was not observed in the study of Hegade et al. 22. Notably, the patients in our study were older and there was a higher percentage of patients with advanced liver disease compared with the Hegadeetal et al. study cohort. This may explain the difference in the results. In addition, two patients in our study received diuretic therapy which might contribute to kidney injury. Therefore, baseline serum creatinine must be measured and monitored closely during fenofibrate treatment, especially in cirrhotic patients or in the case of other risk factors for kidney injury. It may not be appropriate to use fenofibrate in PBC patients with a baseline renal insufficiency.
Our study still had several concerns and limitations. Firstly, 66.7% percent of the enrolled patients were AMA-M2 negative, which is lower than the rate reported in previous studies. The main reasons may be due to the fact that most of patients were hospitalized. The selection bias in hospitalized patients could mean that more AMA negative patients were admitted for a liver biopsy. Secondly, the sample size was relatively small. Therefore, further studies are required to evaluate the long-term efficacy and safety of fenofibrate in PBC patients.
Based on this observational study, we can recommend that fenofibrate could be added to UDCA therapy for PBC patients with a suboptimal response to standard UDCA for at least one year. Periodic monitoring of liver tests should be performed monthly during the first three months and then at three to six month intervals. Renal function should be monitored every three to six months. Fenofibrate therapy should be stopped in patients who develop severe liver injury or fenofibrate-related nephrotoxicity.
In summary, fenofibrate add-on therapy is effective for improving ALP and -GT levels in non-cirrhotic and cirrhotic PBC patients with a suboptimal response to UDCA. However, careful monitoring of liver injury and potential nephrotoxicity should be implemented in fenofibrate-treated PBC patients, especially those with cirrhosis.