Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.10 Madrid oct. 2018
https://dx.doi.org/10.17235/reed.2018.5652/2018
SPECIAL ARTICLE
Quality indicators for endoscopic retrograde cholangiopancreatography. The procedure of endoscopic retrograde cholangiopancreatography
1Unidad de Endoscopias. Servicio de Medicina de Aparato Digestivo. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia. Spain
2Servicio de Calidad Asistencial. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia. Spain
3Servicio de Digestivo. Hospital San Juan de Dios. Santa Cruz de Tenerife. Spain
4Gestión del Conocimiento. SEPD. Madrid. Spain
5Servicio de Medicina de Aparato Digestivo. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia. Spain
INTRODUCTION
Few controlled studies are available about quality in endoscopic retrograde cholangiopancreatography (ERCP). This is paradoxical, since ERCP represents the endoscopic procedure with the highest morbidity and mortality rates, and the one resulting in more lawsuits 1,2.
Gaining insight into the quality level of this or any other issue requires measuring the most relevant aspects of the care we are offering, usually in the form of indicators. Quality indicators may be split into three categories: "structure", "process" and "outcome" 3. "Structure" involves all things related to the steady attributes where health care takes place; "process" includes all that is done for patients and the skill wherewith it is done; "outcome" encompasses all things that benefit patients, all changes in their health status potentially attributed to the care received.
In this context, the SEPD has set out on a project to provide useful quality and safety indicators for digestive endoscopy procedures, adapted to our setting and categorized by levels of evidence 4,5. In this paper quality procedures and indicators are suggested for ERCP.
METHODOLOGY
The present paper, being a part of the aforementioned project by the SEPD 4, was developed along the latter's lines. First, a multidisciplinary team was set up to review the literature and design the ERCP procedure. Their proposal was reviewed by a professional panel selected by the SEPD until a definitive version was reached. Then, indicator tables were developed for these procedures.
Search strategies and study selection
The search and selection strategy was the one used overall for the SEPD project, as detailed in the initial paper 4. Basically, two searches were performed: one for CPGs and one for original and review articles. Furthermore, references quoted in the chosen studies, as well as in the reported significant reviews, and clinical guidelines and meta-analyses, were secondarily reviewed. These references were peer reviewed, and selected when they included recommendations regarding ERCP preparation, execution and monitoring, or structural, process, and/or outcome quality indicators.
Endoscopy procedure design
Based on the literature selected and author experience, procedure-related activities were collected and sorted out. Procedures common to all endoscopic explorations and specific techniques used for specific situations were not included. The result was diagrammed in a parallel flow chart. Group proposals were reviewed and revised by a panel of SEPD-selected professionals until a definitive version was finalized.
Indicator construction
To obtain valid indicators, the quality of the knowledge available on procedure-related activities and the documents selected following the search was assessed. To this end, the GRADE evidence quality grading system was used, where quality is scored as high, moderate, low and very low 6,7.
To ensure reliability and facilitate indicator calculations, each indicator is accompanied by a form including the following: area of application (procedures where it applies), name, formula, type, timing (pre-procedure, procedure, post-procedure), related quality dimension, justification, exclusions and clarifications, and supporting level of evidence.
Indicators common to all digestive endoscopy procedures, which are discussed in a different paper, are excluded 4, albeit supplementary remarks are included for some of them because of their ERCP-specific features.
RESULTS AND DISCUSSION
ERCP procedure
The goal is to maximize procedure quality and safety in order to facilitate patient diagnosis and treatment while optimizing health outcomes (Fig. 1). It includes the following:
1. Patient positioning. Three positions are useful: usually the left lateral oblique-prone position is used, but the standard (left lateral) or prone position may also work.
2. Use of deep sedation or general anesthesia.
3. Duodenoscope check-up. The scope must provide adequate, high-quality viewing, and functions such as tip angulation, elevation, air and water provision, and suction must all work properly.
4. Fluoroscopy equipment preparation and focusing on the right hypochondrium.
5. Duodenoscope lubrication.
6. Duodenoscope insertion:
Blind entry until the pylorus is visualized in the setting sun position.
Left rotation to enter bulb.
Right rotation to enter second portion.
Focus on the papilla.
Turn small knob clockwise and large knob counterclockwise.
Rectify by rotating clockwise.
If unsuccessful, use the long route: further advance the tube to follow the greater gastric curvature until the papilla is in view.
7. Observe and describe abnormalities in papillary area: diffuse mucosal infiltration, papillary conditions, diverticula, polyps, ampulloma. Biopsy collection is indicated in the latter case to facilitate tumor staging.
8. Biliopancreatic cannulation:
Preferably, use a cannulatome or sphincterotome with guidewire to reduce complications.
Direct cannula towards the desired duct: bile duct, between 11 o'clock and 1 o'clock; pancreatic duct, between 1 o'clock and 2 o'clock.
Check location and advance using fluoroscopy (direction of guidewire and contrast medium), trying not to use contrast enhancement until inside of the desired duct.
If access to the desired duct remains unsuccessful after five attempts (difficult cannulation), consider precut. If access to the bile duct fails, consider double guidewire (leave guidewire in pancreas, withdraw sphincterotome and reintroduce with new guidewire), pancreatic stent placement, or termination. In the latter case, the procedure may be attempted later on, or echoendoscopy with rendez-vous or drainage may be considered.
9. Act according to findings:
Leave guidewire in bile duct to secure access and device exchange, and consider sphincterotomy. Also:
- In case of suspected or visualized choledocholithiasis, consider passing a balloon or using a - - Dormia basket. Should this fail, consider balloon sphincteroplasty or stent placement. - Tumor stenosis: sampling and stenting. - Non-tumor stenosis: dilation with/without stenting. - Fistula or bile leakage: sphincterotomy with/without stenting.
If a stricture is found in the pancreatic duct, use dilation with/without stenting. Remove any stones present using a balloon or basket.
If in diagnostic doubt, direct or indirect cholangioscopy may be considered.
10. Withdraw tube. Maneuver slowly and aspirate air and gastric contents as you go.
11. End procedure. Withdraw tube completely and hand over to assistant for cleaning and preparation. Consider antibiotic prophylaxis.
Indicators
A total of 26 indicators (Table 1) have been included, of which seven refer to structure (common to all procedures), 17 refer to process, and two refer to outcome. The complete list of indicators is included in Table 2, but we shall only discuss the following because of their technique-specific status: process-pre-procedure (antibiotic prophylaxis and procedure difficulty grading [Schutz]); process-procedure (deep ductal cannulation in native papilla, common bile duct stone removal, stricture resolution, and radiation estimation). Two outcome indicators have also been developed for adverse events such as post-ERCP pancreatitis and post-ERCP bleeding, as these are highly specific and have an impact on clinical workload.
No indicator for ERCP volume per site, as recommended by some guidelines, has been included as we deem it indicative of quantity rather than quality. Furthermore, such indicator represents an attempt to measure endoscopist experience or training, an aspect already accounted for by indicators common to all endoscopic procedures 4.
Table 1 Quality indicators for endoscopic retrograde cholangiopancreatography (those elaborated upon in the text appear in boldface type)

Numbering is consecutive to that established for colonoscopy markers 5 since this is a single project.
Indeed, not all indicators have the same weight. In fact, the American Society for Gastrointestinal Endoscopy (ASGE) de-fined as priority ERCP indicators those essential to obtain good results, including the following: appropriate indication, duct cannulation, stone removal, and post-ERCP pancreatitis 8.
It should be born in mind that in the GRADE system, which is used to assess the evidence supportive of indicators, "high-quality" evidence includes well-designed randomized studies, which are scarce in this setting. As a consequence, level of evidence is scored on both ends of the scale ("very high" or "low"), and "very high" levels are mainly obtained from clinical guidelines. It is because of this that many of our indicators match the ones therein. Randomized studies with moderate level of evidence are scarce, as are also other designs.
For clarification, indicator numbers (Table 1) are consecutive to those used in the prior two papers for general 4 and colonoscopy 5 indicators.
B.07. Antibiotic prophylaxis
Some clinical guidelines recommend antibiotic prophylaxis in some cases, including known or likely biliary obstruction, biliary or pancreatic fistula, pancreatic pseudocysts or necrosis, liver-transplanted immunosuppressed patients, and active bacterial cholangitis 8,9. A Cochrane 2010 review concluded that prophylactic antibiotics reduce bacteremia and seem to prevent cholangitis, pancreatitis, and sepsis in patients undergoing elective ERCP, although their benefits may be less obvious in the subgroup of patients with uncomplicated ERCP 10. In a recent review 11, the ASGE did not recommend antibiotic prophylaxis in the absence of suspected biliary obstruction or when complete biliary drainage is envisaged; they did recommend it otherwise and for liver-transplanted individuals. Appropriate antibiotics should cover biliary flora (including enterococci and Gram-negative germs).
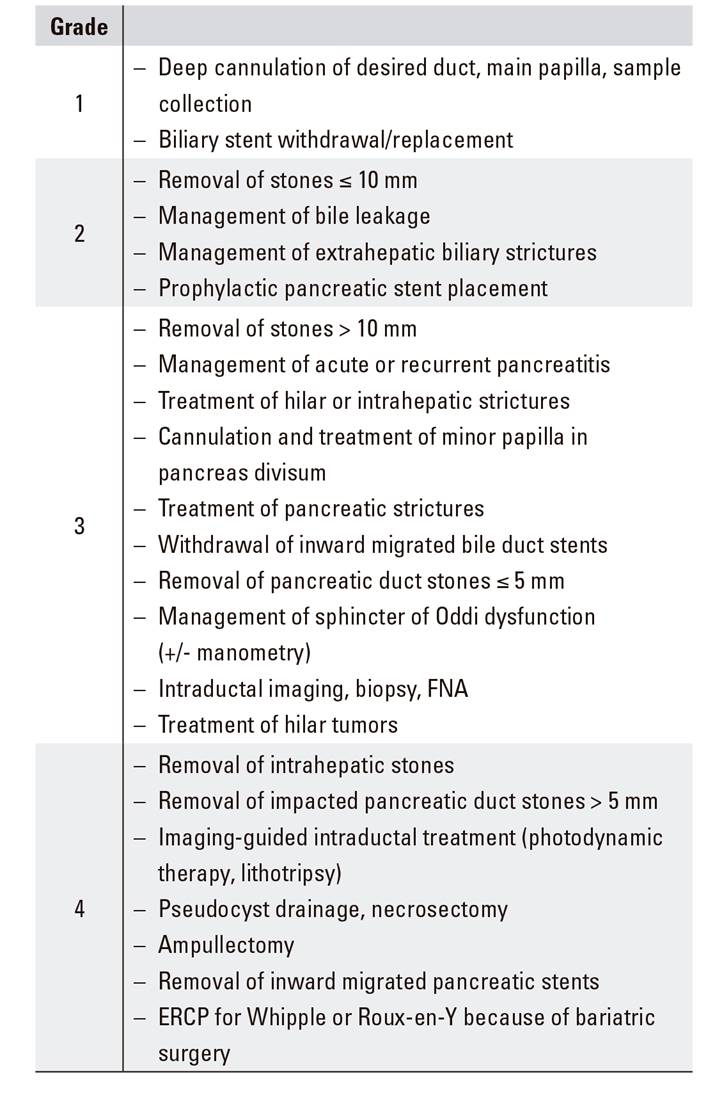
B.08. Procedure difficulty assessment (Schutz grading)
To gain insight into the effectiveness of a procedure such as ERCP, it is key that the expected odds of success be known. In this regard, Schutz published a scale in an attempt to provide expected success data according to expected procedure difficulty (Table 2) 12. Later, a Dutch registry analyzed the influence of such classification, recorded for one year using a standardized system (Rotterdam Assessment Form for ERCP [RAF-E]) 13, on procedure success, and established its relationship with difficulty levels (75.2%, 84.3%, 88.1%, respectively, for grades 3, 2, and 1), albeit within narrow ranges.
Endoscopists with lower numbers of procedures should likely perform grade-1 procedures exclusively, leaving those categorized as grades 2 and 3 to higher-volume institutions 14,15. In this respect, the ASGE, based on this scale, has put forth a 4-grade scale with specific recommendations (levels 1 and 2 for basic endoscopists, levels 3 and 4 for experts) (Table 3) 16.
C.11. Deep cannulation of the desired duct in a native papilla
Successful cannulation of the desired duct (bile, pancreatic or both) is the foundation of the procedure's diagnostic and/or therapeutic success 8,15, hence should be recorded in all cases. Cannulation is deemed as satisfactory when the guidewire passes through the papilla into the desired duct so that it may be advanced deeper and a contrast medium may be injected to visualize the entire duct system. All types of precut are included when the standard technique fails in order to avoid repeat endoscopic procedures or percutaneous cholangiography.
This indicator is restricted to patients with normal papillary anatomy, and also excludes:
Failures because of inadequate sedation, presence of gastric contents, prior abdominal surgery such as pancreatoduodenectomy, gastrojejunostomy and hepaticojejunostomy, and antral or proximal duodenal obstruction, including duodenal deformities from pancreatitis, edema, etc.
Patients with prior sphincterotomy.
Reference to a potential standard is based on task force agreements and the results of a meta-analysis including prospective studies, which found a percentage of 89.3% (95% CI: 0.866-0.919) for bile duct cannulation, and of 84% for pancreatic duct cannulation regardless of institution type 17. Furthermore, cannulation odds have been seen to be dependent on endoscopist experience: for ≤ 50 ERCPs/endoscopist/year, 84.2%; for > 50 ERCPs/endoscopist/year, 91% (mean, 89%) (p < 0.001) (18). Since most patients included in those studies were seen at reference sites, we decided to slightly reduce ASGE-established standards (> 90%) 8 as they are excessively optimistic and do not reflect the conditions extant in our setting.
A point of interest is difficult biliary cannulation, which the ESGE 19 defines as failed cannulation after five attempts, after five minutes, or after more than one pancreatic duct cannulation 20. However, it is clear how this definition may apply to this deep cannulation indicator.
C.12. Choledocholithiasis extraction
Where this test is undertaken in order to remove stones, its success or failure should always be recorded, with adequate information on size and location, as well as on the potential presence of strictures or anatomical changes, including post-surgical ones 21.
This indicator is restricted to cases with smaller stones and normal anatomy, but this proportion may also be useful for other, more challenging stones. Within the scope of its restricted use, over 90% of stones should be successfully extracted with recourse to sphincterotomy and a balloon or basket when appropriate.
As is the case with cannulation, data from the meta-analysis show that extraction is successful in 88.3% (95% CI: 0.825-0.941) of cases 17. This is why we decided, as for cannulation, on the use of reduced, more realistic standards as compared to those established by the ASGE (> 90%) 8,15. Meta-analysis results, however, probably encompass all types of lithiasis regardless of size 17.
Situations may be found that increase complexity and, besides stone size, also relevant are associated anatomical changes, strictures, unfeasible basket use or lithotripsy, and Mirizzi syndrome 22.
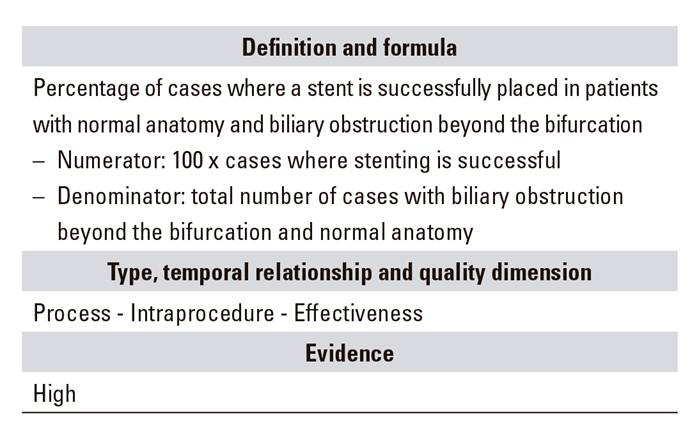
C.03. Stenosis resolution
When the procedure is aimed at stenting for biliary obstruction, the resulting success or failure, and whether it involved sphincterotomy 23 or precut in order to access the bile duct, should always be recorded 8,15, providing adequate information on indication, obstruction location, and presence or absence of strictures or anatomical changes, including post-surgical ones.
The indicator is restricted to the aforementioned cases but may also be useful for the rest. Placing a stent in a biliary obstruction distal to the hilum is considered as a relatively easy procedure, and an essential one for obstructive jaundice, particularly when associated with cholangitis.
As regards the cases it is limited to or indicated for, stenting should be successful in over 90% of them 8,15, and meta-analyses have found success rates of 97.5% (95% CI: 0.967-0.984) 17. Only stenosis dilation would be inappropriate as an indicator because of its high relapse rate.
C.14. Radiation estimation
Fluoroscopy use is inherent in ERCP, this being the source of the latter's radiation exposure risk (both for patients and healthcare providers), which is similar to other radiographic studies 24. Hence, measuring fluoroscopy time and including such measurement as a quality indicator has been suggested by various guidelines for more than ten years now 8,25. The potential to optimize radiation usage without lessening effectiveness supports its inclusion 26.
However, the correlation between fluoroscopy time and radiation dosage is notoriously poor 27, hence other measurements should be used for this estimation whenever possible, including the dose area product (DAP) 24. Various studies support the notion that received radiation dosage may be reduced without modifying fluoroscopy time 24.
E.02. Adverse effects
Fewer complications have been shown to develop when endoscopists perform over 50 procedures per year 18.
E.02.1. Adverse effects: post-ERCP pancreatitis
By consensus, post-ERCP pancreatitis (PEP) 28 is any newly developed or aggravated abdominal pain that is consistent with clinical pancreatitis, occurs beyond 24 hours after ERCP, is associated with an increase in serum amylase (and/or lipase) three times above normal levels, and requires hospitalization for at least two days 29. This concept is highly significant since enzyme changes have been witnessed in up to 75% of patients following ERCP 30. Its incidence is 3.5% (1.6% to 15.7%) 31. While these meta-analysis-derived figures are real, a selection of very high-risk indications (suspected sphincter of Oddi dysfunction) should be considered to establish more appropriate values. Guidelines exhibit no consensus on acceptable standards 8,15, but we should aim at below 7%. Several factors have been shown to increase both pre-procedure and intraprocedure risk 29 (Table 4).
Methods have been devised to reduce its incidence 32: appropriate patient selection and indications, prophylaxis with rectal indomethacin 33,34, use of pancreatic stents in case of initial pancreatic duct cannulation 35 and guidewire cannulation.
E.02.2. Adverse effects: post-ERCP bleeding
Bleeding risk during ERCP ranges from 0.5% to 5% 36, with 1% established as the most approximate figure 37), nearly always associated with endoscopic sphincterotomy 38.
It is defined as the presence of clinical complaints or blood extravasation into the gastrointestinal tract 39, and one should wait for 2-3 minutes after sphincterotomy to assess it 40. Furthermore, more severe delayed bleeding has also been described with melena, hemoglobin drops, or transfusion needs within ten days after ERCP. This type of bleeding is more easily associated with antithrombotic agents.
Balloon sphincteroplasty is safe, and its use without sphincterotomy is recommended for patients with coagulation disorders or juxtadiverticular papilla 36.
Table 5 36 summarizes ERCP-related bleeding risk factors. Use of antithrombotic drugs stands out as the primary factor, although aspirin has not been shown to increase bleeding, hence sphincterotomy could be safely performed in patients on aspirin 41,42,43.
In summary, ERCP is an interventionist technique, and associated quality indicators with evidence of a high success rate in the cannulation of the desired duct (bile, pancreatic, both) include choledocholithiasis extraction and stenosis resolution. Also strong is the measurement of adverse effects, primarily post-ERCP pancreatitis and sphincterotomy-related bleeding.
BIBLIOGRAFÍA
1. Conklin LS, Bernstein C, Bartholomew L, et al. Medical practice in gastroenterology. Clin Gastroenterol Hepatol 2008;6(6):677-81. DOI: 10.1016/j.cgh.2008.02.047 [ Links ]
2. Cotton PB. Twenty more ERCP lawsuits: why? Poor indications and communications. Gastrointest Endosc 2010;72(4):904. DOI: 10.1016/j.gie.2010.01.058 [ Links ]
3. Donabedian A. Basic approaches to assessment: what to assess. Exploration, structure, process and outcomes - Quality assessment and monitoring. Vol. 1. Michigan: Health Administration Press Ann Arbor; 1980. pp. 79-122. [ Links ]
4. López-Picazo J, Alberca-de-las-Parras F, Sánchez-del-Río A, et al. Quality indicators in digestive endoscopy: introduction to structure, process, and outcome common indicators. Rev Esp Enferm Dig 2016;109(4):435-50. DOI: 10.17235/reed.2017.5035/2017 [ Links ]
5. Sánchez-del-Río A, Pérez-Romero S, López-Picazo J, et al. Indicadores de calidad en colonoscopia. Procedimiento de la colonoscopia. Rev Esp Enferm Dig 2018;110(5):316-26. DOI: 10.17235/reed.2018.5408/2017 [ Links ]
6. Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches. The GRADE Working Group. BMC Health Serv Res 2004;4(1):38. DOI: 10.1186/1472-6963-4-38 [ Links ]
7. Atkins D, Briss PA, Eccles M, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res 2005;5(1):25. DOI: 10.1186/1472-6963-5-25 [ Links ]
8. Adler DG, Lien II JG, Cohen J, et al. ASGE/ACG Task Force on quality in endoscope. Quality indicators for ERCP. Gastrointest Endosc 2015;81:54-66. DOI: 10.1016/j.gie.2014.07.056 [ Links ]
9. Alkhatib AA, Hilden K, Adler DG. Comorbidities, sphincterotomy, and balloon dilation predict post-ERCP adverse events in PSC patients: operator experience is protective. Dig Dis Sci 2011;56:3685-8. DOI: 10.1007/s10620-011-1830-8 [ Links ]
10. Brand M, Bizos D, O'Farrell PJR. Antibiotic prophylaxis for patients undergoing elective endoscopic retrograde cholangiopancreatography. Cochrane Database System Rev 2010;10:CD007345. DOI: 10.1002/14651858.CD007345.pub2 [ Links ]
11. Khashab MA, Chithadi KV, Acosta RD, et al. ASGE Standards of Practice Committee. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2015;81(1):81-9. DOI: 10.1016/j.gie.2014.08.008 [ Links ]
12. Schutz SM, Abbott RM. Grading ERCPs by degree of difficulty: a new concept to produce more meaningful outcome data. Gastrointest Endosc 2000;51:535-9 DOI: 10.1016/S0016-5107(00)70285-9 [ Links ]
13. Ekkelenkamp VE, Koch AD, Haringsma J, et al. Quality evaluation through self-assessment: a novel method to gain insight into ERCP performance. Frontline Gastroenterol 2014;5:10-6. DOI: 10.1136/flgastro-2013-100334 [ Links ]
14. Cotton PB. Income and outcome metrics for the objective evaluation of ERCP and alternative methods. Gastrointest Endosc 2002;56(Suppl 6):S283-90. DOI: 10.1016/S0016-5107(02)70026-6 [ Links ]
15. Baron T, Petersen B, Mergener K, et al.; ASGE/ACG Taskforce on Quality in Endoscopy. Quality indicators for endoscopic retrograde cholangiopancreatography. Am J Gastroenterol 2006;101:892-7. DOI: 10.1111/j.1572-0241.2006.00675.x [ Links ]
16. Cotton PB, Eisen G, Romagnuolo J, et al. Grading the complexity of endoscopic procedures: results of an ASGE working party. Gastrointest Endosc 2011;73(8):868-74. [ Links ]
17. DeBenedet AT, Elmunzer BJ, McCarthy ST, et al. Intraprocedural quality in endoscopic retrograde cholangiopancreatography: a meta-analysis. Am J Gastroenterol 2013;108(11):1696-704;quiz 705. DOI: 10.1038/ajg.2013.217 [ Links ]
18. Kapral C, Duller C, Wewalka F, et al. Case volume and outcome of endoscopic retrograde cholangiopancreatography: results of a nationwide Austrian benchmarking project. Endoscopy 2008;40:625-30. DOI: 10.1055/s-2008-1077461 [ Links ]
19. Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48(7):657-83. DOI: 10.1055/s-0042-108641 [ Links ]
20. Halttunen J, Meisner S, Aabakken L, et al. Difficult cannulation as defined by a prospective study of the Scandinavian Association for Digestive Endoscopy (SADE) in 907 ERCPs. Scand J Gastroenterol 2014;49:752-8. DOI: 10.3109/00365521.2014.894120 [ Links ]
21. Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010;71(1):1-9. DOI: 10.1016/j.gie.2009.09.041 [ Links ]
22. Maple JT, Ikenberry SO, Anderson MA, et al. The role of endoscopy in the management of choledocholithiasis. Gastrointest Endosc 2011;74(4):731-44. DOI: 10.1016/j.gie.2011.04.012 [ Links ]
23. Banerjee N, Hilden K, Baron TH, et al. Endoscopic biliary sphincterotomy is not required for transpapillary SEMS placement for biliary obstruction. Dig Dis Sci 2011;56:591-5. DOI: 10.1007/s10620-010-1317-z [ Links ]
24. Kachaamy T, Harrison E, Pannala R, et al. Measures of patient radiation exposure during endoscopic retrograde cholangiography: beyond fluoroscopy time. World J Gastroenterol 2015;21(6):1900-6. DOI: 10.3748/wjg.v21.i6.1900 [ Links ]
25. Romagnuolo J, Cotton PB. Recording ERCP fluoroscopy metrics using a multinational quality network: establishing benchmarks and examining time-related improvements. Am J Gastroenterol 2013;108:1224-30. DOI: 10.1038/ajg.2012.388 [ Links ]
26. Barakat MT, Thosani NC, Huang RJ, et al. Effects of a brief educational program on optimization of fluoroscopy to minimize radiation exposure during endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol 2017;S1542-3565(17)30937-0. DOI: 10.1016/j.cgh.2017.08.008 [ Links ]
27. Stecker MS, Balter S, Towbin RB, et al. Guidelines for patient radiation dose management. J Vasc Interv Radiol 2009;20:S263-73. DOI: 10.1016/j.jvir.2009.04.037 [ Links ]
28. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37:383-93. DOI: 10.1016/S0016-5107(91)70740-2 [ Links ]
29. Kachaamy TA, Faigel DO. Improving ERCP quality and decreasing risk to patients and providers. Expert Rev Gastroenterol Hepatol 2013;7(6):531-40. DOI: 10.1586/17474124.2013.824703 [ Links ]
30. Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc 2004;59:845-64. DOI: 10.1016/S0016-5107(04)00353-0 [ Links ]
31. Cotton PB, Garrow DA, Gallagher J, et al. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc 2009;70:80-8. DOI: 10.1016/j.gie.2008.10.039 [ Links ]
32. Anderson MA, Fisher L, Jain R, et al. Complications of ERCP. Gastrointest Endosc 2012;75(3):467-73. DOI: 10.1016/j.gie.2011.07.010 [ Links ]
33. Montaño Loza A, Rodríguez Lomelí X, García Correa JE, et al. Effect of the administration of rectal indomethacin on amylase serum levels after endoscopic retrograde cholangiopancreatography, and its impact on the development of secondary pancreatitis episodes. Rev Esp Enferm Dig 2007;99:330-6. [ Links ]
34. Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 2012;366:1414-22. DOI: 10.1056/NEJMoa1111103 [ Links ]
35. Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy 2010;42:842-53. DOI: 10.1055/s-0030-1255781 [ Links ]
36. Alberca de las Parras F, Egea Valenzuela J, Carballo Álvarez F. Riesgo de sangrado en la colangiopancreatografía retrógrada endoscópica: impacto del uso de fármacos antitrombóticos. Rev Esp Enferm Dig 2017;109(3):202-10. DOI: 10.17235/reed.2017.4358/2016 [ Links ]
37. Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781-8. DOI: 10.1111/j.1572-0241.2007.01279.x [ Links ]
38. Glomsaker T, Hoff G, Kvaløy JT, et al; Norwegian Gastronet ERCP Group. Patterns and predictive factors of complications after endoscopic retrograde cholangiopancreatography. Br J Surg 2013;100(3):373-80. DOI: 10.1002/bjs.8992 [ Links ]
39. Huibregtse K, Katon RM, Tytgat GN. Precut papillotomy via fine-needle knife papillotome: a safe and effective technique. Gastrointest Endosc 1986;32:403-5. DOI: 10.1016/S0016-5107(86)71921-4 [ Links ]
40. Balmadrid B, Kozarek R. Prevention and management of adverse events of endoscopic retrograde cholangiopancreatography. Gastrointest Endosc Clin N Am 2013;23(2):385-403. DOI: 10.1016/j.giec.2012.12.007 [ Links ]
41. Veitch AM, Baglin TP, Gershlick AH, et al. Guidelines for the management of anticoagulant endoscopic procedures and antiplatelet therapy in patients undergoing endoscopic procedures. Gut 2008;57;1322-9. DOI: 10.1136/gut.2007.142497 [ Links ]
42. Becker RC, Scheiman J, Dauerman HL, et al; American College of Cardiology and the American College of Gastroenterology. Management of platelet-directed pharmacotherapy in patients with atherosclerotic coronary artery disease undergoing elective endoscopic gastrointestinal procedures. Am J Gastroenterol 2009;104:2903-17. DOI: 10.1038/ajg.2009.667 [ Links ]
43. Alberca de las Parras F, Marín F, Roldán Schilling V, et al. Manejo de los fármacos antitrombóticos asociados a los procedimientos endoscópicos. Rev Esp Enferm Dig 2015;107:289-306. [ Links ]
Received: April 16, 2018; Accepted: July 01, 2018











 texto en
texto en