INTRODUCTION
Salivary gland tumors represent 3-10 % of all head and neck neoplasms1. About 80 % of the salivary gland tumors occur in the parotid gland2. Surgical intervention is the mainstay of treatment for parotid gland tumors3).
The parotid gland is closely related to the facial nerve. Facial palsy (FP) is one of the most serious complications that can occur in parotid gland surgery and the primary concern of both surgeons and patients. In the literature, the incidence of transient facial weakness after parotid surgery ranges from 10 % to 68 %, and long-term dysfunction ranges from 0 % to 19 %4.
It would be advantageous to be able to predict preoperatively the possibility of iatrogenic FP or the need for facial nerve sacrifice in parotidectomy, with the aim of a better surgical planning and informed consent. Several risk factors have been identified for postparotidectomy facial nerve dysfunction, including older age, diabetes, malignancy, larger tumor size, tumors involving the deep lobe of the parotid gland and revision surgery5 6-7. However, the described risk factors vary considerably from study to study. In addition, medical comorbidities, such as diabetes and hypertension, were not included in some of those studies, which complicated the retrospective analysis of risk factors for postparotidectomy FP.
The purpose of this study was to determine possible predictive factors related to the occurrence of FP after parotidectomy in the surgical treatment of parotid gland tumors.
MATERIAL AND METHODS
This article is a descriptive and longitudinal study. This study is in accordance with the ethics standards of the institute where it was carried out and with the Helsinki declaration.
Patients
The authors performed a retrospective analysis of cases of parotidectomy in the treatment of parotid tumors carried out at a Portuguese Oncology Institute. Data was obtained from 1991 to 2015. This time frame was selected to provide adequate follow-up of at least 1 year. Patients with pre-operative FP by previously surgery or malignant tumor infiltration were excluded from the study.
Surgical team
Eight different senior surgeons were involved in the surgeries. The surgical team was characterized by an elevated homogeneity in technical abilities. In result, the factor surgeon experience was not included in the statistical analysis.
Surgical procedure
After identifying the main trunk of the facial nerve, the overlying parotid tissue is meticulously elevated from the facial nerve. Sequential branching of the nerve is sequentially dissected, until the entire superficial lobe of the parotid gland lying lateral to the facial nerve is delivered. This completes a superficial or lateral parotidectomy. If a total parotidectomy is indicated, the procedure is extended by meticulous dissection of the main trunk and the branches of the facial nerve from the underlying parotid tissue in a gentle and atraumatic fashion. This allows the salivary tissue lying deep to the facial nerve to be delivered while preserving the nerve and its function.
Superficial parotidectomy was the approach used in cases of fine-needle aspiration cytology suggestive of benignity with tumor limited to the superficial lobe of the parotid gland. We do not support minimal parotid surgery as tumor enucleation.
Total parotidectomy was performed in cases of fine-needle aspiration cytology suggestive of malignancy, in benign tumor originating within or with extension to the deep lobe and in parotidectomy totalization by tumor recurrence in the remaining gland tissue.
Facial nerve function was monitored via visual control by an assistant surgeon. Electromyographic nerve monitoring was not routinely used.
Facial nerve weakness
Clinical evaluation of both preoperative and postoperative facial nerve function was performed via visual control. Postoperative FP was defined as any objective facial weakness in a given area (frontal, zygomatic, buccal, marginal, cervical). A persistent FP was defined by any degree of facial weakness persisting longer than 6 months postoperatively. Postoperative electromyography and electroneurography were not routinely performed.
Variables analysed
The cases were analysed for socio-demographic data (age, sex), patient's comorbidities (hypertension, vascular arterial disease, diabetes, dyslipidemia, obesity), surgical procedure (laterality, superficial/total parotidectomy, primary/revision surgery, concomitant cervical lymph node dissection, enlargement of the excision to the skin), histopathology (benign/malignant, tumor dimension) and clinical outcome (involuntary FP/facial nerve sacrifice, involved territory of the face, face motility recovery). The dimensions evaluated (tumor's length and depth) were obtained from histopathological reports and not from clinical staging to give a more precise value.
Statistical analysis
The authors performed a descriptive analysis of the results, using the mean and SD for continuous variables and frequency for categorical variables. The statistical analysis was performed using Stata 14.0. Spearman's correlation coefficient (rho) was used to evaluate possible factors related to iatrogenic FP. The significance level was set at p < 0.05.
RESULTS
The study sample consisted of a total of 166 patients. There were 80 female (48.2 %) and 86 male (51.8 %) patients, with a mean age of 56.9 years (range 11 to 95 years, SD 16.8).
The frequency of the different comorbidities assessed in the sample was as follows: hypertension 31.9 % (n = 53); vascular arterial disease 15.7 % (n = 26); diabetes 12.1 % (n = 20); dyslipidemia 7.2 % (n = 12); obesity 7.2 % (n = 12).
The surgical procedure was performed on the right parotid gland in 82 (49.4 %) and on the left in 84 (50.6 %) cases. Superficial parotidectomy was performed in 114 (68.7 %) and total parotidectomy in 52 (31.3 %) of the cases. In 18 cases (10. 8%), the surgery was a revision procedure. Concomitant cervical lymph node dissection was performed in 15.1 % (n = 25). The excision was extended to the skin in 7.2 % (n = 12).
Histopathology revealed a benign in 124 (74.7 %) and malignant tumor in 42 (25.3 %) cases. Pleomorphic adenoma, followed by Warthin's tumor, was the most frequent diagnose (Table 1). The mean tumor size (largest axis) was 2.8 cm (range: 0.2 to 10 cm, SD 1.7). The mean tumor thickness was 1.8 cm (range: 0.5 to 5.7 cm, SD 1.1).
In 62 cases (37.4 %), some degree of FP was observed post-operatively. Of these, 12.9 % (n = 8) resulted from facial nerve sacrifice by malignant tumor infiltration (therapeutic indication) (Figure 1).
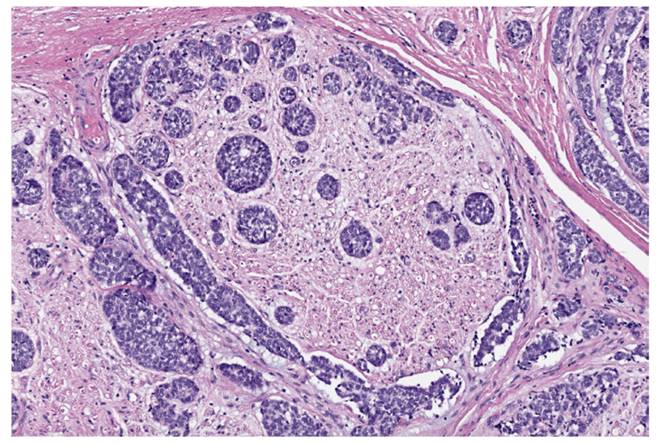
Figure 1. Micrograph showing a segment of facial nerve with perineural invasion of mucoepidermoid carcinoma. H&E stain.
In the remaining 87.1 % (n = 54), the injury of the facial nerve was involuntary and not detected during the procedure (FP not predicted intra-operatively). In these cases, the territory of the marginal branch was the most frequently involved (67.2 %).
In cases of facial nerve sacrifice by malignant tumor infiltration, it was performed when feasible intra-operatively repair with nerve cable grafting. The donor nerve used was usually the greater auricular nerve or, in alternative, the sural nerve.
In the group of involuntary (not detected) injury of the facial nerve with post-operatively FP, the patients started high dose steroids and tailored facial exercises. Complete recovery was observed in 77.8 % (n = 42/54) of the patients. In only 5.6 % (n = 3/54) there was any recovery. Considering the total study sample, excluding the cases of facial nerve sacrifice, temporary facial nerve dysfunction was observed in 26.6 % (n = 42/158), whereas persistent FP occurred in 7.6 % of the cases (n = 12/158).
The extension of surgery (rho = 0.177; p = 0.023) and tumor size (rho = 0.159; p = 0.045) showed correlation with iatrogenic facial nerve injury. The other factors analysed were not found to be statistically significant risk factors (Table 2).
The factors malignancy of the lesion (rho = 0.568; p < 0.001), extension of the surgery (rho = 0.485; p < 0.001) and enlargement of the excision to the skin (rho = 0.7211; p < 0.001) correlated with the facial nerve sacrifice (Table 3).
DISCUSSION
An accurate informed consent is an important part of the preoperative process in any surgery. FP is one of the most serious complications that can occur in parotid gland surgery. It would be advantageous to be able to predict preoperatively the possibility of iatrogenic FP or the need for facial nerve sacrifice in parotidectomy, with the aim of a better surgical planning and informed consent.
The exact mechanism of postparotidectomy FP in the presence of anatomically intact facial nerve is still poorly understood. Neural elongation was proposed as the most probable factor involved in facial nerve dysfunction after surgery of the parotid gland. Peripheral nerves have been found to follow a peculiar stress-strain curve with zones of straightening and elastic elongation, followed by mechanical rupture. Nerve elongation of 6 % seems to cause perineurium tears with disturbance of the intrafascicular homeostasis and unrecoverable loss of the compound action potencial (neurotmesis). After this type of lesion the nerve remains grossly normal, although not functional, which may justify the absence of injury perception by the surgeon. On the other hand, in less significant neural elongation there is no disruption of the endoneurium (neuropraxia or axonotmesis), occurring complete recovery of the nerve function after time8. In our series, there was complete recovery of the face motility in 77.8% of the patients with postoperative FP not resulting from facial nerve sacrifice. So, our results suggest that most cases of FP postparotidectomy are transient and reversible as result of neuropraxia or axonotmesis by neural stretching during the dissection.
In this series, similarly to other studies, the marginal branch was the most frequently involved after parotidectomy. This facial nerve branch seems to be more prone to injury during parotidectomy. Possible reasons that make the marginal branch more liable to be injured and stretched during surgery include the relatively fewer connecting anastomoses and its thinner diameter and longer tracts embedded in the parotid gland. Furthermore, some parotid tumors, such as Warthin tumor, have a tendency to be located in the lower pole of the parotid gland and, therefore, in the path of this facial nerve branch9.
Previous reports identified different risk factors for postparotidectomy FP5 6-7. In our series, contrary of what we might expect, only the extension of surgery and tumor size showed correlation with iatrogenic facial nerve injury. A total parotidectomy, with excision of the deep lobe of the parotid gland, requires a more extensive dissection of the facial nerve with an increased risk of neural elongation and mechanical rupture. We performed total parotidectomy in all malignant tumors, even if low-graded, in order to address the possible intraglandular spread of lymph node metastases, due to the anatomical and oncological importance of the deep parotid lymph nodes10,11. On the other hand, larger tumor size showed to be associated with a more intimate relationship between the facial nerve and the tumor, with increased risk of nerve stretching, and possible tumor extension to the deep lobe of the parotid gland. These risk factors should be taken in account in the preoperative evaluation and demand a much more careful technique during nerve dissection.
Although not identified in our series, the literature points out as risk factors for postparotidectomy FP older age4), diabetes7 and revision surgery6). Neural regeneration seems to be poorer in aged patients. Animal experiments have shown that axonal regrowth of the facial nerve is delayed and the reinnervation pattern is pathological altered in aged individuals12. Motor nerve conduction velocity and amplitude are lower in diabetic patients compared with nondiabetic patients. Also, in diabetic patients, the Schwann cells and myelin sheaths of nerves are much more prone to damage than in nondiabetic patients13. The correlation observed in some studies between revision surgery and long-term facial deficits might be explain by the greater risk of nerve stretching in revision parotidectomy cases when freeing the fine nerve branches from the surrounding scar tissue. Additionally, it is often difficult to differentiate scar tissue from small nerve fibers6.
Sacrifice of the facial nerve is indicated in the presence of preoperative FP and tumor encasement or infiltration. Facial nerve dissection should always be macroscopically radical, avoiding macroscopic residual tumor. To best to our knowledge, we have found no previous studies addressing the predictive factors of facial nerve sacrifice. The factors malignancy of the lesion, extension of the surgery and enlargement of the excision to the skin correlated with the facial nerve sacrifice. Malignancy of the lesion requires a more aggressive surgical approach. In addition, malignant lesions may be associated with tumor's adherence and facial nerve infiltration. Enlargement of the excision to the skin, as far as we know, was not evaluated in previous studies. This procedure is required to perform a complete tumoral excision in the presence of a malignant tumor with skin extension, and reflects a more extensive and aggressive tumor. These factors should be considered preoperatively for a more accurate informed consent about the possibility of facial nerve sacrifice in patients without preoperative FP.
In the present study, electromyography (EMG) was not routinely used. Considering the total study sample and excluding the cases of facial nerve sacrifice, persistent FP was present in 7.6 %, which is within the range of long-term FP described in the literature using EMG14). In fact, the value of intraoperative EMG during parotidectomy is controversial. Previous literature data suggest the benefit of neuromonitoring in minimizing risk of iatrogenic nerve injury. In contrast, authors refer poor sensitivity of intraoperative electromyographic facial nerve monitoring, with facial nerve injury being correctly predicted only in one fourth of the cases and EMG remaining silent during the surgery in 75 % of patients with postoperative FP15 . Others studies, concluded that EMG in primary parotid surgery, in addition to visual facial observation, not diminish the incidence of postoperative FP, not advocating the standardized use of facial nerve monitoring in parotid surgery16. Application of standardized operation procedure and a high level of the operative surgeons' experience may explain the absence of increased risk of FP without intraoperative EMG.
The exact significance of risk factors of FP postparotidectomy remains controversial, because the inconsistency of findings between the different studies. Data should be confirmed by prospective studies with larger number of patients that would provide the statistical power to detect associations that may be difficult to detect in smaller cohorts or by meta-analysis.
As limitations of the present study we point out its retrospective nature and be based on the experience of only one tertiary center. Despite these limitations, this is the first study that evaluates the variables concomitant cervical lymph node dissection and enlargement of the excision to the skin for postparotidectomy FP, and studies the predictive factors of facial nerve sacrifice. In addition, cases were reviewed for patient's comorbidities, which could complicate the analysis of the predictive factors of FP after parotid gland surgery.
CONCLUSION
FP is one of the most serious complications that can occur in parotid gland surgery. Recognize preoperatively the risk factors for iatrogenic FP or need for facial nerve sacrifice in parotidectomy is advantageous for a better surgical planning and informed consent.
A total parotidectomy requires a more extensive dissection of the facial nerve with an increased risk of neural lesion. Large tumors may present a more intimate relationship with the facial nerve and extension to the deep lobe of the parotid gland. Malignant tumors require a more aggressive surgical approach and may be associated with need of facial nerve sacrifice for therapeutic purpose.
The factors age, sex, patient's comorbidities (hypertension, vascular arterial disease, diabetes, dyslipidemia, obesity), lesion laterality, revision surgery and concomitant cervical lymph node dissection were not found to be statistically significant risk factors for iatrogenic FP or need for facial nerve sacrifice in parotidectomy.
In conclusion, patients should be informed before parotidectomy that tumors of larger size and excision of the deep lobe of the parotid gland are risk factors for transient and long-term facial deficits. They should also be informed that malignancy of the lesion and greater extension of surgery correlate with facial nerve sacrifice.

















