Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Farmacia Hospitalaria
versión On-line ISSN 2171-8695versión impresa ISSN 1130-6343
Farm Hosp. vol.37 no.1 Toledo ene./feb. 2013
https://dx.doi.org/10.7399/FH.2013.37.1.96
ORIGINALES
Stability evaluation of 7% chloral hydrate syrup contained in mono and multi-dose bottles under room and refrigeration conditions
Evaluación de la estabilidad de un jarabe de hidrato de cloral al 7% en envases mono y multidosis bajo condiciones ambiente y de refrigeración
C. Bustos-Fierro1, M. E. Olivera2, P. G. Manzo3 y Álvaro F. Jiménez-Kairuz2
1Farmacia Central, Hospital Nacional de Clínicas, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba, Santa Rosa, Argentina
2UNITEFA-CONICET, Departamento de Farmacia, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Haya de la Torre y Medina Allende, Ciudad Universitaria, Córdoba, Argentina
3Centro de Química Aplicada CEQUIMAP, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Haya de la Torre y Medina Allende, Ciudad Universitaria, Córdoba, Argentina
ABSTRACT
Purpose: To evaluate the stability of an extemporaneously prepared 7% chloral hydrate syrup under different conditions of storage and dispensing.
Methods: Three batches of 7% chloral hydrate syrup were prepared. Each batch was stored in 50 light-resistant glass containers of 60 mL with child-resistant caps and in two bottles of 1000 mL to simulate two forms of dispensing, mono and multi-dose, respectively. Twenty five mono-dose bottles and a multi-dose bottle of each batch were stored under room conditions (20 ± 1oC) and the rest of the samples were stored in the fridge (5 ± 2oC). The physical, chemical and microbiological stability was evaluated for 180 days. Stability was defined as retention of at least 95% of the initial concentration of chloral hydrate, the absence of both visible particulate matter, or color and/or odor changes and the compliance with microbiological attributes of non-sterile pharmaceutical products.
Results: At least 98% of the initial chloral hydrate concentration remained throughout the 180-day study period. There were no detectable changes in color, odor, specific gravity and pH and no visible microbial growth. These results were not affected by storage, room or refrigeration conditions or by the frequent opening or closing of the multi-dose containers.
Conclusions: Extemporaneously compounded 7% chloral hydrate syrup was stable for at least 180 days when stored in mono or multi-dose light-resistant glass containers at room temperature and under refrigeration.
Key words: Chloral hydrate syrup; Sedation; Compounding; Pediatrics; Stability.
RESUMEN
Objetivo: Evaluar la estabilidad de un jarabe extemporáneo de hidrato de cloral al 7% bajo diferentes condiciones de almacenamiento y dispensación.
Métodos: Se prepararon tres lotes de hidrato de cloral. Cada lote se almacenó en 50 contenedores de vidrio resistentes a la luz de 60 ml con tapones de protección infantil y en dos botes de 1000 ml para simular dos formas de dispensación, mono y multidosis, respectivamente. Veinticinco envases monodosis y un envase multidosis de cada lote se almacenaron en condiciones ambiente (20 ± 1oC)y el resto de las muestras se almacenaron en el frigorífico (5 ± 2oC). Se evaluaron las estabilidades física, química y microbiológica durante 180 días. Se definió la estabilidad como la retención de al menos el 95% de la concentración inicial del hidrato de cloral, la ausencia de partículas visibles y de cambios en el color y/o el olor, así como el cumplimiento de los requisitos microbiológicos de los productos farmacéuticos no estériles.
Resultados: Al menos el 98% de la concentración inicial de hidrato de cloral se mantuvo a lo largo de los 180 días del periodo de estudio. No se apreciaron cambios detectables en el olor, el color ni la densidad o el pH y tampoco se apreció crecimiento microbiológico. Estos resultados no se vieron influidos por las condiciones de almacenamiento, estar a temperatura ambiente o refrigerada ni por la frecuencia de apertura y cierre de los contenedores multidosis.
Conclusiones: El compuesto de jarabe de hidrato de cloral extemporáneo al 7% fue estable durante al menos 180 días en envases de vidrio mono o multidosis, resistentes a la luz, a temperatura ambiente y con refrigeración.
Palabras clave: Jarabe de hidrato de cloral; Sedación; Formulación; Pediatría; Estabilidad.
Introduction
The chloral hydrate is a sedative drug widely used in pediatrics, especially to administer to children before diagnostic procedures such as computerized axial tomography scan and magnetic resonance imaging that requires patient immobility. It is also used in intensive care units, pediatric emergency departments and dental surgery to decrease stress and anxiety caused by technological procedures, light and sound levels and the presence of strangers1,2. It is a cheap, effective and safe drug that can be easily handled and administered when performing nasofibroscopy3. Chloral hydrate is quickly absorbed and metabolized into the liver, erythrocytes and kidneys to trichloroethanol (active metabolite) and to trichloroacetic acid (inactive metabolite). The binding to the proteins of the active metabolite is 70-80% and its half-life is from four to twelve hours. The rapidity with which it induces sleep (30 to 60 min) is attributed to the active principle, while the long-lasting action (4 to 8 hours) is due to its active metabolite. A sedative dose (50-100 mg/Kg) has minimal effects on breathing and blood pressure and the reflexes are slightly suppressed. The patient can, therefore, be fully awake. Chloral hydrate is generally well tolerated when a single dose is given1. It has a low toxicity as long as the recommended dose is not exceeded and it is administered for a short period of time4. Chloral hydrate as sleep inductor for electroencephalogram recordings in children of one to five years old of age is more effective than midazolam5,6.
It achieves a significantly deeper level of sedation compared with other sedative agents and children remain calmer when undergoing echocardiography7.
Chloral hydrate occurs as transparent colorless crystals, with a penetrating slightly acrid odor and a slightly bitter caustic taste. It is highly soluble in water. Because of its unpleasant taste and its irritant action on the gastric mucosa, oral administration of dilute solutions is advisable8,9. From the chemical point of view chloral hydrate is a gemdiol, near infrared studies, in aqueous solution, showed the existence of a labile equilibrium between the gemdiol and a dimolecular 1:1 complex of the aldehyde and water (eq. 1)10. Besides, it has been proposed that, in slightly acid or neutral aqueous solutions, chloral hydrate decomposes by an oxidation-reduction process forming dichloroacetaldehyde, trichloroacetic acid and hydrochloric acid (eq. 2). Exposure to light and heat can speed up this degradation process10.

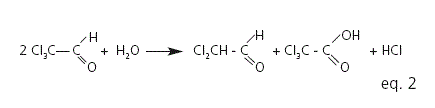
On the other hand, it has been described that chloral hydrate decomposes in diluted aqueous solution of sodium hydroxide (eq. 3) by first-order kinetics catalyzed by hydroxyl ions10.

At present, there is no commercially available formulation of chloral hydrate, thus it is considered an orphan drug. Because of this shortage, it is prepared as an extemporaneous formulation in hospital pharmacies11. The 10% oral solution of chloral hydrate is codified by USP 30 Ed.12, whereas the 7% chloral hydrate syrup is codified by the Argentine Pharmacopoeia 6th Ed13. It is known that, for its texture and palatability, properly flavored syrups effectively mask taste and they are greatly preferred by pediatric patients14. In our Institution, the 7% chloral hydrate syrup is prepared and stored in 100, 250, 500 and 1000 mL of multi-dose light-resistant glass containers. The main production demand of the syrup has increased significantly from 6.4 L/year during 2009 and 2010 to 20 L/year during 2011, which forced us to prepare larger batches to be stored in the pharmacy until dispensed. This situation points out the need for stability studies which allow establishing adequate storage conditions and an expiration date based on stability studies. Notice that the frequent opening of the multi-dose bottles during the utilization period could increase the concentration of sugar and of chloral hydrate due to water evaporation. Besides, aqueous solution of 10% chloral hydrate produces a pH of 3.5-5.5 15 which can lead to hydrolysis (inversion) of sucrose, with the subsequent loss of the syrup consistency and the possibility of microorganism fermentation.
Few stability studies of liquid formulations of chloral hydrate have been carried out11,16. However, both stability studies on 7% chloral hydrate syrup nor multidose containers considering the possible opening and closing of the containers were found, and they were not designed to assign an expiration date or to define optimal storage conditions. In a previous study it has been proposed that the decrease in the pH of the formulations of chloral hydrate can be used as an indicator of the degradation of the active principle16.
In this context, the objective of this work is to evaluate the physical, chemical and microbiological stability of 7% chloral hydrate syrup both under room and refrigeration conditions in mono and multi-dose bottles.
Materials and Methods
Chloral hydrate (C2H3CI302, USP grade, Parafarm®, Bs. As., Arg.) was assayed by means of titration, according to FA 8 Ed16. The determinations were performed in quintuplicate which showed a 100.6 ± 0.4% concentration of C2H3CI302. Refined sucrose (Ledesma®, Salta, Arg.), Alcohol 96o (Porta®, Cordoba, Arg.), NaOH 1.0 N and 0.1 N solutions (Anedra®, Bs.As., Arg.) and distilled water were also used.
Preparation of 7.0% chloral hydrate syrup
Three batches of 5.0 L of 7% chloral hydrate syrup, flavored with bitter orange fluid extract 30% (Parafarm®, Bs. As., Arg.) were prepared in accordance with the procedure described in Appendix to observe reproducibility and robustness of the gathering and control methods. Each batch was stored in 50 light-resistant glass containers of 60 mL with child-resistant caps and in two bottles of 1000 mL, with the same characteristics, to simulate two dispensing forms, mono- and multi-dose, respectively.
Stability evaluation
Twenty five mono-dose bottles and a multi-dose bottle of each batch were stored under room conditions in an air-conditioned room and the rest of the samples were stored in the fridge. The temperature of both conditions was daily monitored using a digital thermo-hygrometer, registering values of 20 ± 2oC y 5 ± 2oC for room and refrigeration conditions, respectively. All the samples were labeled and stored for 180 days. For physical and chemical stability, one mono-dose container and an aliquot of 35 mL from the multi-dose containers of each chloral hydrate syrup batch, at the mentioned temperature conditions, were collected on days 0, 7, 15, 30, 45, 60, 75, 90 and 180.
• Physical Stability Evaluation. At each time point, physical stability was assessed by visual examination and by determination of specific gravity, according to USP <841> method 1 using a 25-mL calibrated pycnometer12. Physical stability was defined as the compliance of the specific gravity according to the USP specifications (≥ 1.30 g/mL)12 and the absence of either visible particulate matter, or color and/or odor changes.
• Chemical Stability Evaluation. Chemical stability was assessed following the concentrations of chloral hydrate and HCl in the samples. Chloral hydrate concentrations were determined by titrimetry with 1.0 N sodium hydroxide adapting USP30 Chloral Hydrate 10% Oral Solution assay to 7% syrup. In addition the time required to produce the reaction between chloral hydrate and sodium hydroxide was determined to be 10 min in the syrup instead the 2 min described for the 10% oral solution. Besides, the syrup samples were measured by weight instead of volume to avoid errors due to its high viscosity. Briefly, approximately 27.7 g of 7% chloral hydrate syrup were weighed in an appropriate glass-conical flask. Then, 25.0 mL of sodium hydroxide were added and mixed for 10 min. Finally, 4 drops of 1% phenolphthalein alcoholic solution were added. The excess of sodium hydroxide, regarded as (A), was immediately titrated with 1.0 N sulfuric acid (Anedra®, Bs.As., Arg.). For the second titration, 5.55 g of the syrup were weighed in a 100 mL conical flask and titrated with 0.1 N sodium hydroxide solution after adding 10 drops of 1% phenolphthalein alcoholic solution. This solution was regarded as (B). The weight in mg of chloral hydrate in the amount of 7% chloral hydrate syrup taken for the first titration was calculated by the formula:

A coefficient variation of 0.47% for this method was obtained from 10 replicated titrations.
For pH determinations, the samples were thermostatized at 25oC in a water bath. The pH values were recorded with an ADWA AD 8000 pH-meter, with an Ag/AgCl-reference electrode, calibrated at the same temperature with commercial reference buffer solutions of pH 4.01 and 7.00 (HACH®, USA).
The stability-indicating capability of the titration and pH determination methods was also evaluated in this work. An aliquot of 1.0 M HCl was added to three 100 mL samples of 7% chloral hydrate syrup to get HCl concentrations equivalent to 1 and 5% of chloral hydrate degradation, according to equation 2. The resulting pHs were 2.50 ± 0.09 and 2.05 ± 0.09, for HCl concentrations equivalent to 1 and 5% of chloral hydrate degradation, respectively. The chemical stability of the formulation was defined as not less than 95% of the initial drug concentration remaining in the samples and a pH value not less than 2.05. Results are expressed as the mean of three batch determinations for each storage condition.
• Microbiological stability evaluation. Microbiological assessment of chloral hydrate syrup, only on the samples stored under the most unfavorable conditions (multi-dose containers at room temperature), was carried out on days 0, 30, 60, 90 and 180. The samples were subjected to microbiological evaluation in order to determine if they meet the microbiological attributes of non-sterile pharmaceutical products which is set as total aerobic microbial count below 102 cfu/mL, total combined yeasts/molds count below 2 cfu/mL and absence of Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and Salmonella spp. Limit content of microorganisms was performed according to USP <1111>12 and ANMAT No 7667 disposition18.
Results and discussion
Table 1 shows some relevant properties of the three batches of 7% chloral hydrate syrup. All the test samples were practically colorless and free of visible particles. Table 2 shows the chemical and physical stability descriptors after six months of assay. In addition, no particles were observed in the bulk solution or in the container closures. Besides, little or no chloral hydrate loss occurred in any of the samples at any storage temperature throughout the study and there was no significant change in pH since it remained within the range of 2.97 to 3.09 which suggests that there was no chemical degradation. Specific gravity after 6 months of storage under refrigeration and room temperature complies with USP specifications. Accordingly, no increase in concentration of chloral hydrate due to vehicle evaporation was observed in the multi-dose containers even after 6 months of storage, and in spite of having been opened 9 times for sampling during the stability experiments.
Microbiological tests were negative in the three batches which prove that the product complies with official specifications after 6 months of storage.
All these aspects support the conclusion that, for at least 180 days, 7% chloral hydrate syrup is chemically, physically and microbiologically stable under refrigeration and room temperature. Besides, the frequent opening of the multi-dose containers for the withdrawal of successive portions had no impact on chloral hydrate concentration or the syrup features.
These results are in agreement with those reported in a previous work 14 which informed that both 10% chloral hydrate aqueous solution and syrup did not show significant changes after 3 month of storage at room temperature or 60oC. Several hospital centers report in their formularies that chloral hydrate solutions, prepared as extemporaneous formulations at concentrations between 5 and 10% of chloral hydrate, had an expiration date between 15 and 30 days at most19-22.
The shorter expiration date can probably be a safety concern since these formulations may lack studies to document stability11,14,22.
Conclusions
This study provides conclusive information about the period and the storage conditions in which chloral hydrate syrup can be utilized as a sedative in pediatrics.
A 7% chloral hydrate in a sucrose-based, orange flavored syrup, in light-resistant glass containers of 1000 and 60 mL can be stored for at least 180 days. The three batches under study at 20oC and 5oC showed excellent physical stability as well as practically no loss in chloral hydrate concentration. Levels of HCl as degradation product were well below the acceptable limits. Therefore, hospital pharmacies, can set an expiration date of 6 months under any of the conditions studied.
Acknowledgements
We thank all the staff that works at the Main Pharmacy of the National Hospital of Clinics and, particularly, the pharmacists Gisela Bertolotto and María Emilia Gavelli for their important collaboration.
References
1. Campo Angora M, Albiñana Perez MS, Ferrari Piquero JM, Herreros de Tejada López-Coterilla A, López-Coterilla A. Utilización del hidrato de cloral en pediatría. Usos clínicos, preparaciones galénicas y experiencia en un hospital. Farm Hosp. 1999;23:170-5. [ Links ]
2. Open database: Medline Plus. Hidrato de Cloral (citado 07-06-2012). Disponible en: http://www.nlm.nih.gov/medlineplus/spanish/druginfo/meds/a682201-es.html. [ Links ]
3. Patrocinio JA, Patrocinio LG. Nasofibroscopía en los niños: dificultades y como facilitar su realización. En: Sih T, Chinski A, Eavey R, editores. IV Manual de Otorrinolaringología Pediátrica de la IAPO (Interamerican Association of Peditric Otorhinolaryngology). SP, Brazil: Lis Gráfica & Editora; 2006;135-140 (citado 07-06-2012). Disponible en: http://www.iapo.org.br/manuals/23-5.pdf. [ Links ]
4. Uberos Fernández J. Sedación y Radiología. Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria (citado 07-06-2012). Disponible en: http://www.sepeap.org/archivos/revisiones/radiologia/sedacion.htm. [ Links ]
5. López ME, Lopez I, Troncoso L, Avaria MA, Novoa F. Hidrato de cloral y midazolam en sedación para electroencefalograma en niños de 1 a 5 años. Rev Chil Pediatr. 1995;66:204-208. [ Links ]
6. D'Agostino J, Terndrup TE. Chloral hydrate versus midazolam for sedation of children for neuroimaging: a randomized clinical trial. Pediatr Emerg Care. 2000;16:1-4. [ Links ]
7. Wheeler DS; Jensen RA; Poss WB. A randomized, blinded comparison of chloral hydrate and midazolam sedation in children undergoing echocardiography. Evidence portal, Virtual Health Library, Bireme - PAHO - WHO, 2001. (citado 02-05-2012). Disponible en: http://pesquisa.bvsalud.org/evidences/resources/CN-00373455. [ Links ]
8. Real Farmacopea Española. 2da. Edición. 1ra reimpresión corregida, Ministerio de Sanidad y Consumo, Madrid, España; 2003. [ Links ]
9. Sociedad Argentina de Pediatría. Cloral, Hidrato de (citado 18-06-2012). Disponible en: http://www.sap.org.ar/staticfiles/cd_neo/drogas/c/c8.htm. [ Links ]
10. Fairbrother JE. Chloral hydrate. In: Florey K, ed. Analytical profiles of drugs substances. Vol. 2. New York: Academic Press Editorial; 1973:85-143. [ Links ]
11. Taguchi M, Horiuchi T, Mimura Y, Adachi I. Studies on Hospital Preparation, "Chloral Hydrate Syrup". Jap J Hosp Pharm. 1999;25: 546-551. [ Links ]
12. USP30-NF25. 2007. The United States Pharmacopeia-The National Formulary. Rockville MD. The United States Pharmacopeial Convention, Inc. [ Links ]
13. Farmacopea Argentina 6ta Edición. 1978. Comisión Permanente para la Farmacopea Argentina. Ministerio de Salud de la Nación. Argentina. [ Links ]
14. Parasrrampuria J. Liquid Oral Preparations. In: Swarbrick J, ed. Encyclopedia of Pharmaceutical Technology. 2nd edition, New York: Marcel Dekker Inc; 2002:1674-1685. [ Links ]
15. WHO-The International Pharmacopoeia. 2nd Supplement of Fourth Edition. 2011 (citado 14-08-2012). Disponible en: http://apps.who.int/phint/en/p/docf/. [ Links ]
16. Kakehi K, Nakano M, Nishiura S, Tachibana S, Ishida S, Taneda M. Examination of the stability of chloral hydrate and its preparation by capillary electrophoresis. Yakugaku Zasshi. 1999;119: 410-6. [ Links ]
17. Farmacopea Argentina. 8va Edición. 2012. Comisión Permanente para la Farmacopea Argentina. Ministerio de Salud de la Nación. Argentina (citado 14-05-2012). Disponible en: http://www.anmat.gov.ar/webanmat/fna/octava_edicion/Segundo_Volumen.pdf. [ Links ]
18. Disposición Nacional: Calidad microbiológica de los productos farmacéuticos no obligatoriamente estériles. Disposición no. 7667, Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (ANMAT), Ministerio de Salud de la Nación, Secretaría de Políticas, Regulación e Institutos, Buenos Aires, Argentina (2010). Disponible en: www.anmat.gov.ar/boletin_anmat/octubre_2010/Dispo_7667-10.pdf. [ Links ]
19. Farmacia Hospitalaria. Hospital Materno-Infantil Carlos Haya. Catálogo de fórmulas no estériles. 3th Versión; Málaga, España. 2010 (citado 15-05-2012). Disponible en: www.carloshaya.net/LinkClick.aspx?fileticket=ZGNkhfjbhmI%3D&tabid=435. [ Links ]
20. Alvarez Rabanal V. Cuadernos de Formulación Magistral. Fichas de Formulación. Palma de Mallorca, España (citado 15-05-2012). Disponible en: http://usuarios.multimania.es/magistralia/HIDRA-TODECLORAL100mgmljarabe.html. [ Links ]
21. Ministerio de Sanidad y Consumo. Formulario Nacional. Hidrato de Cloral Oral Indicaciones. Madrid, España (citado 15-05-2012). Disponible en: www.sefh.es/ficherospediatria/Hidratodecloral100mgmljarabe.doc. [ Links ]
22. Del Arco J. Centro de Información del Medicamento. Colegio Oficial de Farmacéuticos de Bizkaia, España (citado 15-05-2012). Disponible en: http://www.svnp.es/Documen/formumagis.pdf. [ Links ]
![]() Correspondence:
Correspondence:
E-mail: alfejika@gmail.com
(Álvaro F. Jiménez-Kairuz)
Recibido: 7 de octubre de 2012
Aceptado: 28 de diciembre de 2012














