Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Farmacia Hospitalaria
versão On-line ISSN 2171-8695versão impressa ISSN 1130-6343
Farm Hosp. vol.39 no.4 Toledo Jul./Ago. 2015
https://dx.doi.org/10.7399/fh.2015.39.4.8883
Oral chemotherapy: food-drug interactions
Administración de citostáticos vía oral: interacciones fármaco-alimento
Sara Santana Martínez, José Antonio Marcos Rodríguez and Elia Romero Carreño
Pharmacy Department. Hospital Universitario Virgen Macarena, Seville (Spain).
ABSTRACT
Introduction: oral chemotherapy is increasingly used in Oncology. It has important advantages. such as patient comfort. but it also brings new challenges which did not exist with the intravenous therapy. Some of these drugs have interactions with food. leading to changes in their bioavailability. As they are drugs of narrow therapeutic margin. this can lead to alterations in their efficacy and/or toxicity.
Objectives: A. Assessing the level of knowledge on the administration of oral cytostatics that present restrictions with meals (drugs that have to be taken with/without food) among the outpatients. B. Minimizing the incorrect administration and the risk of food-drug interactions. providing patients with information as to how and when drugs have to be administrated.
Methods: once the oral cytostatics with food restrictions were identified. we asked the patients in treatment about the information they had received from the doctor and the way they were taking the medication. We provided those who were taking the drug incorrectly with the right information. In the following visit. it was confirmed if the patients that had been previously taking the cytostatic incorrectly. were taking them in a correct way (intervention accepted/not accepted).
Results and conclusions: 40% of the patients interviewed used to take the drug incorrectly. We detected a great diversity depending on the dispensed drug. 95% of the 39 interventions made were accepted. The data obtained suggest the need to reinforce the information that the patient receives. It is important to make sure that the patient understands how and when the oral cytostatic should be administered.
Key words: Oral chemotherapy; Food-drug interactions; Pharmaceutical care.
RESUMEN
Introducción: el uso de citostáticos orales está cada vez más extendido en oncología. Presenta ventajas importantes, como la comodidad para el paciente, pero también supone nuevos retos que no se planteaban con la terapia intravenosa. Algunos de estos fármacos presentan interacciones con los alimentos, dando lugar a cambios en su biodisponibilidad. Al tratarse de fármacos de estrecho margen terapéutico, pueden dar lugar a alteraciones en su eficacia y/o toxicidad.
Objetivos: evaluar el nivel de conocimiento sobre el modo de administración por parte de los pacientes que acuden a la consulta de pacientes externos de oncohematología del hospital de aquellos citostáticos orales que presentan alguna restricción respecto a su consumo con alimentos (deben tomarse o bien en ayunas. o bien con alimentos). Minimizar al máximo la administración incorrecta de los citostáticos dispensados y el riesgo de que se produzcan interacciones con los alimentos, proporcionando información a los pacientes acerca del modo correcto de administración.
Material y métodos: una vez identificados los citostáticos orales con restricciones respecto a su consumo con alimentos, además de la información aportada por farmacia, se preguntó a los pacientes la información que habían recibido por parte del médico acerca de cómo debía administrarse el fármaco, el modo en que se lo tomaban finalmente y, en caso de no hacerlo adecuadamente, se les reforzó la información pertinente. En el siguiente ciclo se confirmó si efectivamente el paciente se lo administraba correctamente, en caso de hacerlo previamente de forma incorrecta (intervención aceptada/no aceptada).
Resultados y conclusiones: un 40% de los pacientes entrevistados se administraban el fármaco incorrectamente. Los resultados muestran una gran diversidad en función del fármaco dispensado. Se realizaron un total de 39 intervenciones, que fueron aceptadas en un 95%. Los datos obtenidos sugieren la necesidad de reforzar la información que el paciente recibe más allá de la primera visita para asegurarnos de que ha comprendido las condiciones en las que el fármaco debe administrarse.
Palabras clave: Quimioterapia oral; Interacción fármaco-alimento; Atención farmacéutica.
Introduction
In recent years, with the objective of making chemotherapy administration easier, the most active lines of oncologic research have directed their efforts towards the development of oral cytostatic agents (Halfdanarson and Jatoi, 2010). Currently, the administration of oral chemotherapy represents a major focal point among Oncologists, as shown in the increase of oral cytostatics available during recent years (Stuurman et al, 2013). According to a 2010 estimate by the National Comprehensive Cancer Network (NCCN), an alliance of the 25 leading oncology centres in the world, by 2013 the oral administration of cytostatics will have reached a 25% of all cytostatic agents administered (Halfdanarson and Jatoi, 2010).
Therefore, even though the majority of antineoplastic treatments are still administered parenterally, oral administration is becoming firmly established within first line treatments for certain carcinomas, as is the case of capecitabine for metastatic colorectal cancer, because it has been demonstrated that disease free survival and overall survival, as well as toxicity profiles, are not different to those of intravenous treatments (Cassidy et al., 2011), and there is an additional convenience in oral administration, and the risk of intravenous administration is avoided. That is why the new oral antineoplastic drugs, with mechanisms of action based on blocking new therapeutic targets or metabolic pathways, represent a therapeutic alternative in constant growth.
Despite representing a very appealing treatment alternative, it is also associated with new challenges which can occasionally restrict its use. The convenience of these treatment regimens for outpatients is a fact, because it does not compromise clinical outcomes; but we must not forget that these drugs have a narrow therapeutic margin, and are often administered in combination with other agents with similar characteristics, and subject to potential interactions with other drugs or food-drug interactions (DFIs). On the other hand, the pharmacodynamic characteristics of drugs can vary over time as a consequence of concomitant treatments or eating habits (Ruggiero et al., 2012).
Drug-Food Interactions
The administration of oral antineoplastic drugs with meals can cause major variations in drug bioavailability (Ruggiero et al., 2012). These changes, which can lead to a reduction in therapeutic activity or an increase in adverse effects, are particularly important in this type of drugs, which present a narrow therapeutic margin.
The direct effects of interactions between diet and oral cytostatics are primarily of pharmacokinetic nature, while their indirect effects would be pharmacodynamic (Jiménez Torres et al., 2009). Pharmacokinetic interactions due to the concomitant administration of food and drugs are the most common, and will lead to alterations in drug absorption, distribution, metabolism, and excretion (Singh and Malhotra, 2004). These pharmacokinetic effects have an impact on drug bioavailability, and therefore can have a significant effect on the pharmacodynamic properties of the antineoplastic drug, in terms of toxicity and/or efficacy. And because bioavailability has been defined as the amount of drug which reaches the bloodstream and causes a therapeutic effect (Ruggiero et al., 2012), it will be directly affected by the way of administration of the drug. Therefore, optimal bioavailability is essential for cytotoxic agents, because a prolonged exposure to them is key for their antineoplastic activity.
On the other hand, and generally speaking, all patients won't have an identical response to the same type of DFI (Zhang et al., 2005), and therefore pharmacokinetic modifications must be analyzed considering the likelihood that some types of food, by altering the activity of transport or metabolizing enzymes, could modify the antineoplastic response to these drugs (Singh y Malhotra, 2004), and the genetic variability in the enzymatic systems for each individual must also be taken into account. Therefore, such as occurs with drug-drug interactions, the clinical significance of DFIs can present a high variability, low prevalence (1% of the total) and low or no severity in 40% of cases, moderate in 50%, and severe in less than 10% of all DFIs (Jefferson, 1998).
And even though bioavailability studies are a component in the early stages of clinical development of medications with oral administration, DFIs are not clearly defined, classified and characterized in the case of oral antineoplastic drugs (Couris et al., 2000). However, their clinical importance has currently started to become acknowledged, and it has been considered to study them with the same methodology which supports clinical trials (Valle et al., 2005), because it provides the best scientific evidence in early stages (Kuppens et al., 2007). Unfortunately, the information available about more than three hundred food-drug interactions that have been described has not been based on this level of evidence (Jiménez Torres et al., 2009).
Legal regulations for new oral medications, and particularly for those with narrow therapeutic index, demand the demonstration of lack of effect on their efficacy and safety profiles by their intake jointly with food, as well as information about the pharmacokinetic, pharmacodynamic or pharmacogenetic origin of these situations, and their scope in the different population groups. Thus, the FDA recommends conducting bioavailability studies on oral medications, taken with and without food, in order to demonstrate that both administration situations are bioequivalent (FDA, 2003). However, the extent of modification of pharmacokinetic response does not always determine, either linear or proportionally, the severity of the pharmacodynamic modification; and in this sense, it is accepted that pharmacodynamic response or clinical relevance are less documented than pharmacokinetic modifications. Besides, in case that no bioequivalence could be demonstrated, it must be explained that these changes in the drug won't translate into pharmacodynamic changes in patients, or interfere with the efficacy and safety profile of the treatment. Indeed, there are some examples of drugs which can illustrate this situation: in the case of gefitinib, a mean increase in Cmax (maximum plasma concentration of the molecule) of 37% will only translate into a 6% increase in adverse effects in patients (Jiménez Torres et al., 2009). Overall, DFIs present with high variability in their clinical response, which makes it difficult to associate them with treatment failure or toxicity in patients.
Despite the low incidence and apparent low clinical relevance of DFIs, it is important to understand and control the administration of this type of drugs by patients, in order to identify potential causes leading to lack of efficacy by the drug, and consequently its poor efficiency and/or development of toxicity which might represent the discontinuation of medication, or placing the patient's life at risk.
Materials and methods
Design of the study
Experimental intervention study conducted in a third level hospital during the period from April to September, 2013. A bibliographic search was conducted for relevant articles, focused on identifying those publications on drug-food interactions with oral chemotherapy, starting with the question "DFIs with marketed oral antineoplastic drugs" in the PubMed database, and using the following key terms: "antineoplastic agents, food effect, oral chemotherapy, food-drug interaction, pharmacokinetics". At the same time, there was a review of the product specifications for all oral antineoplastic drugs dispensed to hospital outpatients, and the potential interactions with food for each one of them was analyzed, using the following databases: Drugs, Bot Plus, Micromedex, Pubmed, UpToDate, and the AEMPS On-Line Drug Information Centre.
A database was created with those drugs which presented some type of food restriction, and the most relevant consequences of the incorrect administration of these drugs regarding food (increase or reduction in AUC, Cmax, bioavailability...) (Table 1).
Data collection
The information which formed the basis for developing this study was obtained through normalized interviews with patients who came to collect their medication to the Onco-Haematologic Outpatient Unit. In the interview, patients were asked about the information they had received from the physician about drug administration, and the way they were taking it and, in case this was incorrect, the Pharmacist provided correct information about the way of administration, according to drug interactions. In a subsequent visit by the patient to the Outpatient Unit (the following course of treatment), it was confirmed if those patients that had been previously taking the cytostatic incorrectly, were now taking it in a correct way (intervention accepted/not accepted).
Sample Population and Study Period
Patient selection was conducted with the Landtools® computer program, through its Outpatient Dispensation module used at the Onco-Haematological Outpatient Unit of the Hospital Pharmacy. All those patients on treatment with oral antineoplastic agents who presented some type of food restriction were included.
The data collection period was of 6 months, starting on April 2013 and ending up on September, 2013. Patients who failed to attend the unit for drug collection during that period were interviewed by telephone, in order to confirm whether the intervention had been accepted or not.
Study variables
The main variables in the study were the way of administration by patients, the information received from the prescribing specialist, and the acceptance of pharmacist intervention.
The information provided by the prescribing physician about the manner of administration of the drug regarding meals, and the way of drug administration by the patient, were considered correct when they coincided with the recommendations in the product specification or in different databases.
It was considered that the intervention by the pharmacist was accepted when it corrected the way of administration of medication in those patients in which an incorrect way of drug administration had been detected.
The independent variables studied were sociodemographic and clinical (gender, age, diagnosis), administrative (hospital unit, prescribing physician) and in terms of medication (drug dispensed).
Statistical analysis of outcomes
The descriptive study of the sample was conducted with the SPSS 18.0 statistical analysis program. Outcomes were expressed as percentages, as these are qualitative variables.
Methodological design limitations
For the study to clarify the real scope of DFIs and the consequences of an incorrect administration of medication, it would be necessary to take into account clinical variables, or to monitor the levels of the drugs involved.
Outcomes
In total, 97 patients were interviewed (54% male, 46% female), with a median 65-year-age, ranging from 32 to 88 years. Approximately half of patients (50.51%) were over 65-year-old.
Among those drugs dispensed at the unit, the following antineoplastic drugs with use restricted with meals were detected: abiraterone, capecitabine, erlotinib, etoposide, lapatinib, nilotinib, pazopanib, and temozolamide. Out of these, only one (capecitabine) had to be administered within 30 minutes after a meal. The seven remaining drugs had to be administered without food; that is to say, one hour before or two hours after any food intake (Delgado et al., 1997). Among the most frequent consequences of incorrect administration of the drug, there was an increase in the area under the curve of plasmatic concentrations (AUC), bioavailability (F) and maximum plasmatic concentration (Cmax), which occurred with abiraterone, erlotinib lapatinib, nilotinib, and pazopanib. A reduction in AUC and Cmax was also possible, such as happened with temozolamide.
Two of the drugs, capecitabine and etoposide, had no specific information about the consequences entailed by their incorrect administration.
According to the information collected in the product specifications for capecitabine, its administration with food reduces its speed of absorption, but only modifies to a minimal extent the value of AUC of its active metabolites. On the other hand, the current safety and efficacy data described in its product specifications are based on its administration with food; and therefore, that is how it is recommended to administer the drug.
Regarding etoposide, the oral bioavailability of the drug shows a significant variation among patients. Its product specifications state that it is preferable to administer the etoposide capsules on an empty stomach. On the other hand, there are studies suggesting that, at least when the doses administered are equal to 100mg or higher, the presence of food does not interfere significantly with the drug bioavailability (Harvey et al., 1985).
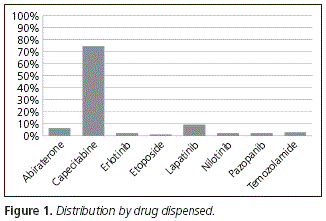
Regarding the oral cytostatic agents collected by patients at the Hospital Outpatient Unit, the main one is capecitabine, which represents 74.23% of the whole, followed by lapatinib (9.28%), abiraterone (6.19%), temozolamide (3.09%), erlotinib, nilotinib and pazopanib, with 2.06% respectively, and etoposide (1.03%) (Fig. 1).
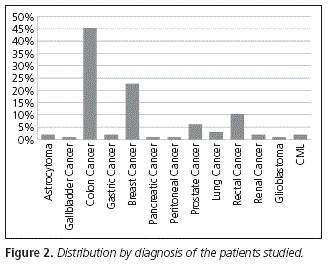
Regarding the diagnosis presented by patients, colon cancer is the main one with 45.36% of the whole sample, followed by breast cancer (22.68%), rectal cancer (10.33%) and prostate cancer (6.18%) (Fig. 2).
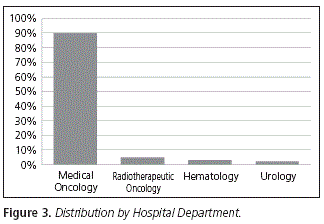
In terms of the hospital unit where the oral chemotherapy agent was prescribed, 89.80% of the whole prescription was conducted in Medical Oncology, followed by Radiotherapeutic Oncology (5.10%), Hematology (3.06%) and Urology (2.04%) (Fig. 3).
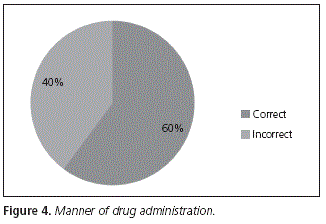
Out of the whole number of patients interviewed, 60% took their medication according to the indications regarding meals in the product specifications, vs. 40% who took it incorrectly (Fig. 4).
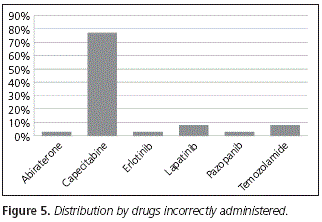
From all the patients who took their medication incorrectly, 77% were patients on treatment with capecitabine; that is to say, these patients did not take the drug within 30 minutes after a meal. Then, 8% were patients on treatment with lapatinib and temozolamide respectively, followed by abiraterone (3%), erlotinib (3%) and pazopanib (3%). No patients taking nilotonib or etoposide incorrectly were detected (Fig. 5).
Therefore, 39 interventions were conducted in that percentage of patients in which incorrect administration of drugs was detected (40%), explaining /reminding to them the correct way of administration. From the whole number of interventions conducted, there was a 95% rate of acceptance among patients (Fig. 6).
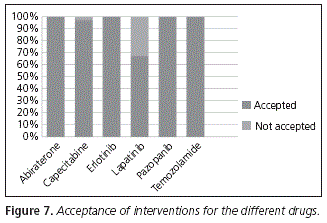
The acceptance of the interventions, classified by each drug for which an incorrect administration was detected, appears in Figure 7. The only drugs for which interventions were conducted and not accepted were capecitabine (with a 97% acceptance rate) and lapatinib (acceptance rate: 67%). The reason for lack of acceptance of the intervention was that the patient on treatment with capecitabine had no breakfast and, therefore, she could not take the drug after the first meal of the day. In the case of the patient with lapatinib, she decided to continue taking the medication with food, because she complained of stomach discomfort when she took it before breakfast.
In the rest of drugs, there was a 100% acceptance rate.
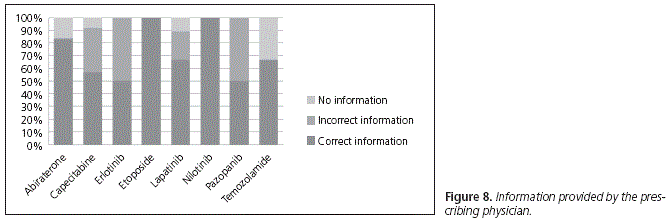
When asked about the information received from the physician about how to take their medication (Fig. 8), 88.33% of patients on treatment with abiraterone answered that they had been given correct information during their visit, while 16.67% reported that they had received no information about it.
In the case of capecitabine, 56.94% of patients had received correct information. From the remaining patients, 34.72% had received incorrect instructions (taking the drug before breakfast, half an hour after meals, or before meals), while 8.33% claimed that they had received no information about the correct way of administration.
Regarding erlotinib, with only two patients on treatment, we found that one of them was taking it correctly, exactly as it had been explained to him in the unit, while the other one had received wrong information (taking it after meals).
Regarding lapatinib, 66.67% of patients had received correct information, 22.22% had received wrong information and took it with food, and 11.11% claimed that they had not been informed about it at the hospital unit.
In the case of etoposide, the only patient on treatment had received correct instructions. It was the same for nilotinib, with two patients on treatment.
On the other hand, one of the two patients on pazopanib was taking it correctly, just as it had been explained to him, while the other one was taking it incorrectly (with meals), following the instructions received.
Finally, 2 of the 3 patients on treatment with temozolamide claimed that they had received the correct information, while one patient said that he had not received any information about it at the hospital unit.
Discussion
Data obtained about the way of drug administration, and information received from the prescribing physician, suggest the need to reinforce the information received by patients, and ensuring that they have understood the circumstances under which the drug must be administered.
We have detected an important proportion of patients who were unaware of the correct way of administration for those oral cytostatics collected from our hospital unit which presented some type of restriction regarding meals (40%). Coinciding with other studies to this respect, we have assessed that medications are occasionally prescribed without taking into account the level of understanding or cooperation by patients (Leal et al., 2004). The outcomes obtained in our study are slightly worse than those described in a previous study conducted in our country, which analyzed the level of knowledge by patients collecting medication from the Hospital Pharmacy, and which showed 69% of patients with a high level of knowledge, 28.6% with an intermediate level, and 2.4% with a low level of knowledge about the drugs dispensed (Santos-Pérez et al., 2012). In our study, the data obtained have varied depending on the drug dispensed. While some patients had received in their majority the correct information (abiraterone, nilotinib, etoposide), in other cases the information received by a major proportion of patients had been incorrect, or they claimed that they had not been informed (capecitabine, erlotinib, lapatinib, pazopanib, temozolamide).
Once the correct information on administration had been provided, the majority of patients took their drug in the correct way (95%). According to other published papers, the involvement by pharmacists in patient training during the administration of medication and discharge from hospital leads to a significantly lower number of medication errors (Hodgkinson et al., 2006). Even though the existing evidence about non-hospitalized patients is not conclusive (Hodgkinson et al., 2006), the data obtained in our study show good results linked with the need to reinforce the information received by patients about the conditions under which the drug should be administered. This would encourage a reduction in the development of drug-food interactions, and therefore increase the efficacy and safety of the therapy.
Finally, it is necessary to highlight the importance and need for multidisciplinary work alongside other specialties, such as Medical Oncology, Radiotherapeutic Oncology, Hematology, Urology, and another group of healthcare professionals such as nurses, in order to select the information that will be provided to patients, which should be simple, truthful and direct. It will be essential to reinforce these administration recommendations in subsequent visits, and to confirm the correct administration.
Bibliography
1. Cassidy J, Saltz L, Twelves C, Van Cutsem E, Hoff P, Kang Y, Saini JP, Gilberg F, Cunningham D. Efficacy of capecitabine versus 5-fluorouracil in colorectal and gastric cancers: a meta-analysis of individual data from 6171 patients. Ann Oncol. 2011;22(12):2604-9. [ Links ]
2. Couris RR, Tataronis GR, Dallal GE, Blumberg JB, Dwyer JT. Assesment of healthcare professionals' knowledge about warfarin-vitamin k drug-nutrient interactions. J Am Coll Nutr . 2000; 19 (4): 439-445. [ Links ]
3. Delgado O, Puigventós F, Serra J. Administración de medicamentos por vía oral. Med Clin. 1997;108:426-35. [ Links ]
4. FDA (Food and Drug Administration). Guidance for Industry: Bioavailability and bioequivalence studies for orally administered drug products. General considerations. Informe del Center for Drug Evaluation and research (CDER). Rockville: FDA; 2003. [ Links ]
5. Halfdanarson TR, Jatoi A. Oral cancer chemotherapy: the critical interplay between patient education and patient safety. Curr. Oncol. Rep. 2010; (12): 247-252. [ Links ]
6. Harvey VJ, Slevin ML, Joel SP, Johnston A, Wrigley PFM. The effect of food and concurrent chemotherapy on the bioavailability of oral etoposide. Br. J. Cancer. 1985; 52: 363-367. [ Links ]
7. Hodgkinson B, Koch S, Nay R. Strategies to reduce medication errors with reference to older adults. International Journal of Evidence-Based Healthcare. 2006; (4): 2-41. [ Links ]
8. Jefferson JW. Drug and diet interactions: avoiding therapeutics paralysis. J Clin Psychiatric.1998; 59: 31-39. [ Links ]
9. Jiménez torres NV, Romero Crespo I, Ballester Solaz m, Albert Marí A, Jiménez Arenas V. Interacciones de los antineoplásicos orales con los alimentos: Revisión Sistemática. Nutr Hosp. 2009; 24(3):260-272. [ Links ]
10. Kuppens IE, Witteven PO, Witteveen PO, Schot M, Schuessler VM, Daehling A, et al. Phase I dose-finding and pharmacokinetic trial of orally administered indibulin (D-24851) to patients with solid tumors. Invest New Drugs. 2007; 25 (3): 227-235. [ Links ]
11. Leal M, Abellán J, Casa MT, Martínez J. Paciente polimedicado: ¿conoce la posología de la medicación?, ¿afirma tomarla correctamente? Aten Primaria. 2004;33(9):451-6. [ Links ]
12. Ruggiero A, Maria G, Coccia P, Mastrangelo S, Maurizi P, Riccardi R. The role of diet on the clinical pharmacology of oral antineoplastic agents. Eur J Clin Pharmacol. 2012; 68:115-122. [ Links ]
13. Santos-Pérez MI, García-Rodicio S, Abajo del Álamo C. Conocimiento de los tratamientos en pacientes hospitalarios: herramienta necesaria para la seguridad asistencial. Rev Calid Asist. 2012; 27(5): 270-274. [ Links ]
14. Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer drugs. Underlying mechanism and implications for oral chemotherapy. Clin Pharmacokinet. 2004; 43 (15): 1127-1156. [ Links ]
15. Stuurman FE, Nuijen B, Beijnen JH, Schellens JHM. Oral Anticancer Drugs: Mechanisms of low bioavailability and strategies for improvement. Clin Pharmacokinet. 2013; 52: 399-414. [ Links ]
16. Valle M, Di Salle E, Jannuzzo MG, Poggesi I, Rocchetti M, Spinelli R, et al. A predictive model for exemestane pharmacokinetics/pharmacodynamics incorporating the effect of food and formulation. British Journal of Clinical Pharmacology. 2005; 59 (3): 355-364. [ Links ]
17. Zhang L, Strong JM, Oiu W, Lesko LJ, and Huang SM. Scientific Perspectives on drug transporters and their role in drug interactions. Mol Pharm. 2005; 3 (1): 62-69. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: sarasolo_@hotmail.com
(Sara Santana Martínez).
Recibido el 21 de febrero de 2015
Aceptado el 23 de abril de 2015











 texto em
texto em