Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Farmacia Hospitalaria
versión On-line ISSN 2171-8695versión impresa ISSN 1130-6343
Farm Hosp. vol.39 no.5 Toledo sep./oct. 2015
https://dx.doi.org/10.7399/fh.2015.39.5.8381
ORIGINALES
Patient safety: prescription of drugs that prolong the QT interval
Seguridad en el paciente: prescripción de fármacos que prolongan el intervalo QT
María Jesús Hernández-Arroyo1, Alfonso Díaz-Madero1 and David Menacho-Miguel2
1Pharmacy Department, Primary Care Management of Zamora, Zamora.
2Community Pharmacy, Salamanca. Spain.
ABSTRACT
Objective: to determine the prescription of drugs with known risk to prolong the QT interval in a Healthcare Area, to provide information to those physicians responsible about the risk factors associated with its development, and to improve patient safety.
Methods: a descriptive cross-sectional observational study of prevalence. A total of 4,964 patients from a Healthcare Area treated in one month with drugs with known risk were included in the study. Risk drugs, interactions and predisposing factors were identified. Physicians were provided with the list of patients with drugs with known risk, recommendations, and a questionnaire to know more risk factors, utility and clinical attitude. A descriptive statistical analysis was conducted.
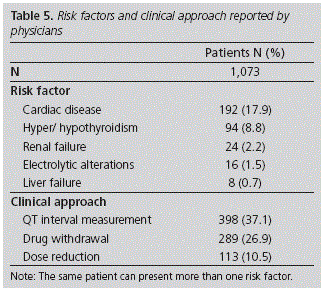
Results: of the total number of patients in the Area, 3.2% were treated with drugs with known risk. 64.0% were women, 57.5% were older than 65 years and 39.6% had drug interactions. The mean number of risk factors per patient was 1.78 (CI 95%: 1.74-1.81). Antidepressants (41.2%) and antibiotics (40.4%) were the most commonly prescribed drugs with known risk. 25.4% of the physicians returned the questionnaire and reported the clinical attitude in 1,073 patients: the drug with known risk was withdrawn in 289, the dose was reduced in 113, and an electrocardiogram was performed in 398. Physicians identified other risk factors: heart disease (17.9%) and hypo/hyperthyroidism (8.8%).
Conclusions: the detected prevalence of prescription of drugs that prolong the QT interval is relevant, considering that the patients also had other risk factors. Their identification can improve the quality of care and patient safety.
Key words: QT interval prolongation; Torsade de pointes; Drug interactions; Pharmaceutical intervention; Patient safety.
RESUMEN
Objetivo: conocer la prescripción de fármacos con riesgo conocido de prolongar el intervalo QT en un área de salud, informar a los médicos responsables de los factores de riesgo asociados a su aparición y mejorar la seguridad del paciente.
Métodos: estudio descriptivo transversal y observacional de prevalencia. Se incluyeron 4.964 pacientes de un área de salud en tratamiento con fármacos con riesgo conocido en un mes. Se identificaron fármacos de riesgo, interacciones y factores predisponentes. Se proporcionó a cada médico los pacientes con fármacos con riesgo conocido, las recomendaciones y la encuesta para conocer más factores de riesgo, su utilidad y su actitud clínica. Se realizó un análisis estadístico descriptivo.
Resultados: el 3,2% de los pacientes del área estaban tratados con fármacos con riesgo conocido. El 64,0% eran mujeres, 57,5% mayores de 65 años, y el 39,6% presentaban interacciones. El número medio de factores de riesgo por paciente fue 1,78. Los fármacos con riesgo conocido más frecuentes fueron antidepresivos (41,2%) y antibióticos (40,4%). El 25,4% de los médicos devolvió la encuesta informando de la actitud clínica en 1.073 pacientes: se retiró el fármaco con riesgo conocido en 289, se redujo la dosis en 113 y se realizó electrocardiograma en 398. Los médicos identificaron otros factores de riesgo: problema cardiaco (17,9%) e hiper/hipotiroidismo (8,8%).
Conclusiones: la prevalencia detectada en la prescripción de fármacos que prolongan el intervalo QT es relevante teniendo en cuenta que los pacientes tenían además otros factores de riesgo. Su identificación permite mejorar la calidad de la atención y la seguridad del paciente.
Palabras clave: Prolongación del intervalo QT; Torsade de pointes; Interacciones farmacológicas; Intervención farmacéutica; Seguridad en el paciente.
Introduction
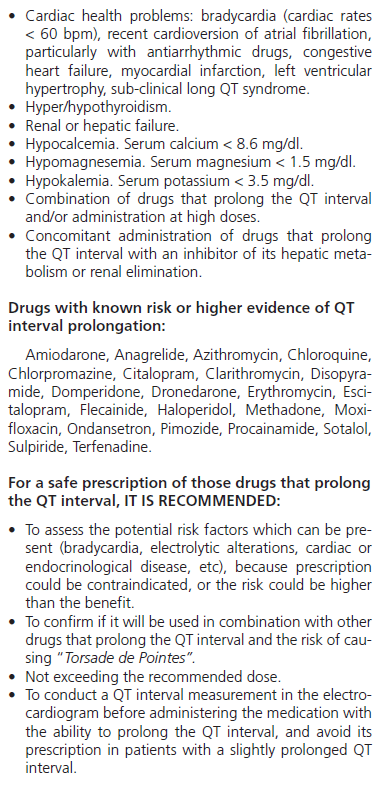
The drug-induced long QT syndrome was first described in 1964, when it was observed that quinidine could prolong the QT syndrome and cause severe arrhythmias1. Though this adverse event was initially associated with antiarrhythmic drugs, there is a continuous increase in the list of drugs which are able to prolong it at therapeutic doses.
Even though measurement through electrocardiogram (ECG) won't determine accurately the arrhythmogenic risk of drugs, generally there is a qualitative relationship between the QT interval prolongation and the risk of torsade de pointes (TdP)2. This is not a frequent adverse event, but the high incidence of associated sudden deaths assigns special importance to it when the medication is used in large populations, or there are safer alternative options3,4,5. Currently, regulatory agencies demand the identification of this potential risk in any new medication before approval6, which doesn't rule out any post-marketing development2, which in fact represents one of the most common causes of restriction of use and/or drug withdrawal from the market3. Recently, the Spanish Agency of Medicines and Medical Devices (AEMPS) has published safety warnings about the risk of QT interval prolongation with medications such as citalopram, escitalopram, ondansetron and domperidone.
Even though it is true that long QT syndrome and TdP are caused primarily by certain drugs, there are many predisposing risk factors frequently existing in the same patient7. Some well-documented factors are: advanced age, female gender, electrolytic alterations (hypocalcemia, hypomagnesemia and hypokalemia), liver or renal dysfunction, previous history of cardiac disease (congestive heart failure, myocardial infarction, bradycardia, left ventricular hypertrophy, and recent cardioversion of atrial fibrillation), or simultaneous treatment with more than one drug which prolongs the QT interval or can inhibit its renal metabolism or elimination8-10. The pharmacological groups most frequently involved are antiarrhythmic, antihistaminic, antimicrobial, antiemetic, neuroleptic, and antidepressant drugs11-13.
The objective of this study is to determine the prevalence of the prescription of drugs with risk of prolonging the QT interval within a Healthcare Area, and to inform those physicians responsible about the risk factors associated with TdP development, with the aim to improve patient safety.
Method
A cross-sectional and observational descriptive study of prevalence which included patients from a Healthcare Area, who were dispensed during December, 2013, through prescription by the National Health System (NHS) some drug with known risk (DKR) of prolonging the QT interval.
The selection of one single month intended to ensure the concomitance of treatments. Patients under 18-year-old were excluded. Data were obtained from the Pharmacy Information System Concylia, which contains information about medicinal products dispensed with NHS prescriptions by the retail pharmacies in Castille and Leon.
In order to identify those medications which might prolong the QT interval, there was a review of the webpage by the Arizona Centre for Education and Research on Therapeutics (AzCERT); www.azcert.org. Medications were classified into 3 levels of risk (known, potential and conditional), based on the level of clinical evidence available14. The drugs selected were marketed in Spain and for out-of-hospital use.
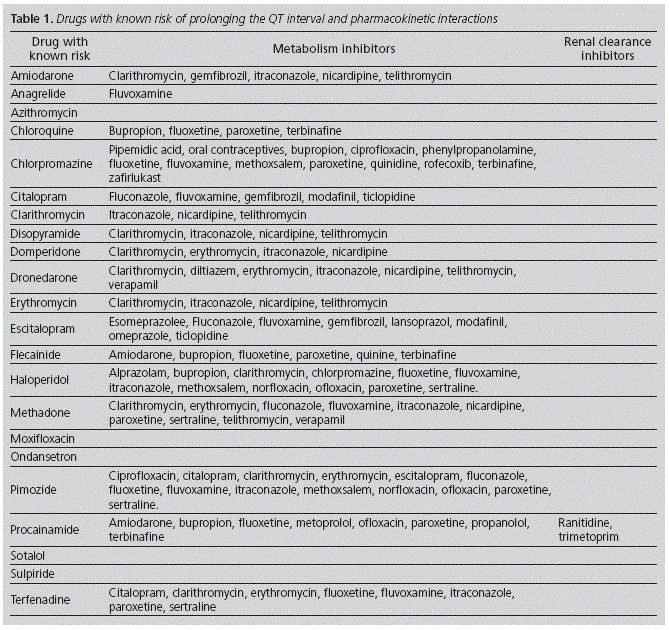
To conduct the search of interactions with DKRs, there was a review of product specifications and the on-line LEXICOMP® platform (Lexi-interact). Pharmacodynamic interaction was defined as that treatment concomitant with other drugs with risk (known, potential or conditional) of prolonging the QT interval. Pharmacokinetic interaction was defined as that treatment concomitant with other drugs with strong inhibition of the enzyme involved in the DKR metabolism, or with other drugs which reduce its renal clearance.
The factors of risk which could boost the prolongation of QT interval and/or cause TdP were the following:
- Obtained from the Concylia program: age ≥65 years, female gender, pharmacodynamic / pharmacokinetic interactions with the DKR, and/or dosing of citalopram or escitalopram superior to that recommended by the AEMPS for patients over 65-year-old (the only drugs for which it is possible to know if the dose of risk is exceeded, according to the presentation dispensed)15,16.
- Obtained from clinical records: cardiac disease (congestive heart failure, myocardial infarction, bradycardia, left ventricular hypertrophy, and recent cardioversion of atrial fibrillation), renal impairment, liver failure, electrolytic alterations (hypocalcemia, hypomagnesemia and hypokalemia), and hyper/hypothyroidism.
In February, 2014, each physician was provided by the Department of Primary Care Pharmacy with information about factors which increase the likelihood of developing long QT syndrome and drug-induced TdP (Annex), as well as the list of patients in their workload on treatment with any DKR. This list included: Patient Identification Code (PIC), age, gender, DKR or drug with risk of prolonging the QT interval, and drugs that can modify its renal metabolism or elimination. Recommendations were also prepared in order to minimize risks, and a survey was designed with the following objectives: to obtain more complete information about risk factors in each patient, clinical attitude, and opinion regarding the utility of the information provided. It was requested that the survey should be returned to the Department of Pharmacy in an anonymous manner, both regarding the physician and the patient. On the other hand, it was analyzed through the Concylia program if, by April, 2014, the DKR had been withdrawn on those patients identified, or replaced by other drug for the same indication but without any proarrhythmic risk or, at least, with lower risk.
The statistical analysis of the study was conducted using the mean value, standard deviation (SD) and confidence interval (CI) of 95% for continuous variables, and percentages for categorical variables.
Outcomes
Twenty-two drugs marketed in Spain were identified with known risk of prolonging the QT interval, as well as thirty-five drugs which inhibited its hepatic metabolism or renal clearance (Table 1).
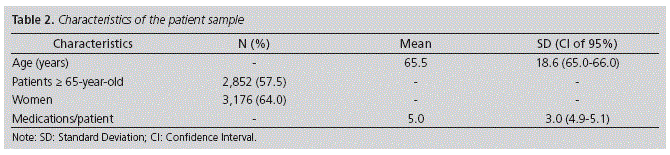
5,574 patients on treatment with at least one DKR were identified. For the study, patients under 18-year-old were excluded (610), and the treatments for 4,964 patients were analyzed; this represents a 3.2% of the total number of users over 18-year-old with a health insurance card for the Healthcare Area. Patient characteristics are shown in Table 2.
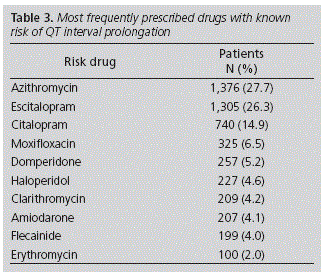
Within this population studied, 5,159 DKRs were recorded. Table 3 shows the 10 drugs more frequently prescribed with high risk of prolonging the QT interval.
The risk factor more frequently identified was female gender (64.0%); 57.3% of these female patients were over 65-year-old.
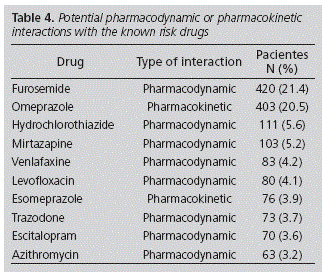
From the whole number of patients, 1,966 (39.6%) were on concomitant treatment with some drug that interacted with the DKR: 1,488 (30.0%) presented potential interactions with other drugs with the ability to prolong the QT interval, 261 (5.3%) presented interactions with drugs that inhibited the DKR's metabolism, and 217 (4.4%) presented both types of interaction. No interactions were detected with drugs that inhibit the renal elimination of the DKR. 2,597 interactions were detected, which represents a mean 1.32 (SD: 0.75; CI 95%: 1.29-1.35) per patient. Out of those 102 patients who presented 3 or more interactions, 76 (74.5%) were women and 86 (84.3%) were ≥65-year-old. It is important to highlight that potential interactions between two DKRs were detected in 138 patients. Those drugs which interacted more frequently with DKRs are shown in Table 4.
The mean number of risk factors detected per patient, without considering those reported by physicians (age ≥65 year-old, female gender, interactions and dosing) was 1.78 (SD: 1.18; CI 95%: 1.74-1.81). 568 (11.4%) patients had no associated risk factors; on the other hand, 1,249 (25.2%) presented 3 or more.
From those 2,045 patients on treatment with citalopram or escitalopram, 200 (9.8%) exceeded the maximum dose recommended by the AEMPS for patients over 65 years of age.
The information about patients on treatment with DKRs and factors which increase the risk of long QT syndrome was communicated to all Primary Care Physicians in the Area (213); 54 (25.4%) out of these returned the completed survey, which allowed to obtain additional information about 1,073 (21.6%) patients (Table 5). 96.3% of physicians considered that receiving information and recommendations about patients in their workload was useful; 40.7% acknowledged that they did not assess any potential risk factors before prescription; and 96.3% considered it was useful to receive information about other risks associated with the use of medications in their patients. The analysis conducted in April revealed that DKRs had been withdrawn to 2,720 (54.8%) patients. For 220 patients, the medication was replaced by another with the same indication, but without risk or lower risk of prolonging the QT Interval (another antibiotic was prescribed to 139 patients, the neuropsychiatric treatment was modified for 65 patients, and antiemetic and antiarrhythmic treatment was modified for 9 and 7 patients, respectively). The pharmacological groups of DKRs more frequently withdrawn or replaced were: antibiotics (60.8%), antidepressants (22.8%), antiarrhythmic drugs (5.6%), antiemetics (5.5%) and neuroleptics (5.0%).
Discussion
It is widely known that some drugs have the ability to prolong the repolarization of ventricular activity potential, which has even led to the withdrawal of medications; but little is known about the frequency with which these are prescribed, alone or in combination with other drugs which can also have a proarrhythmic effect. This fact is even more important in women, advanced age patients, those with a past history of cardiac disease, electrolytic alterations, and renal or liver impairment.
In the present study, the frequency of prescription within one month in a Healthcare Area of drugs with high risk of prolonging the QT interval, both in an isolated way and in combination, has been analyzed. It was found that 3.2% of patients with healthcare insurance within the Area were receiving outpatient treatment with at least one of these drugs. Other authors have found a higher prevalence (10-20%), because the studied periods of time were over one year17,18. 58% of patients were over 65-year-old, and the majority were women, the same as in other series of cases published17-19.
With the analysis conducted, it was intended to identify those patients who could benefit with a closer follow-up, and even with cardiac monitoring through an ECG.
The most frequent pharmacological groups of risk were antibiotics (40.4%) and antidepressants (41.2%); these results are similar to those from other studies in the community setting17,18, with the difference that the macrolide most widely prescribed in this study was azithromycin, while the most prescribed ones in the others were clarithromycin and erythromycin. The reason for this is that azithromycin was not considered as a risk drug, because the Food and Drug Administration notified the warning after its publication20,21. The fact that antibiotics are one of the most frequent groups of risk can be due to their seasonal profile of use, with higher use in months such as the one when the study was conducted, and specifically, azythromycin due to its convenient dosing (3 days of treatment). Regarding antidepressants, the AEMPS has issued informative notes about risk with citalopram and escitalopram, depending on age and dose15,16. In the present study, 200 patients on treatment with these drugs exceeded the maximum recommended dose. Antibiotics and antidepressants are pharmacological groups with high rates of prescription in Primary Care, which shows the importance of conducting an adequate risk-benefit assessment, before prescribing these medications in a risk population.
On the other hand, in the case that a patient is being treated with a DKR and requires the administration of another, the one that presents lower proarrhythmic risk and does not inhibit its renal metabolism or elimination should be selected. In a review of 229 published cases of TdP induced by non-antiarrhythmic drugs, it was found that 39% of cases were caused by the combination of more than one drug with the ability to prolong the QT interval, and 38% by the combination of a drug with the ability to prolong the QT interval and a drug that inhibited its metabolism11. In our study, 39.6% of patients were receiving simultaneous treatment with other drugs with the ability to interact with the DKR, and this outcome was very superior to the ones described by other studies conducted in the United States, which found an incidence around 10%17,18. The explanation could lie in the lack of computerized systems of prescription in our Healthcare Area, which could alert of potential interactions of this type.
The drugs most frequently involved in the interactions detected were diuretics (furosemide and hydrochlorothiazide). The AzCERT considers that both furosemide and hydrochlorothiazide will only represent a risk at high doses, interactions with other drugs, etc, but these are also drugs with a high prescription rate.
As mentioned earlier, the presence of predisposing factors is also essential for the development of severe arrhythmia. In our study, when only data obtained from the Concylia program were considered, 1,249 patients who had 3 or more risk factors were identified, and a mean rate of almost 2 per patient. For this reason, it was considered necessary to collect more information, through a survey targeted at physicians, about the awareness of associated risk, the clinical approach followed after assessment (withdrawal of the drug, dose reduction, and/or performing an ECG), identification of other predisposing factors, and opinion about the utility of this type of information). From the 54 physicians who returned the survey, practically all considered that the information was useful, and approximately 40% acknowledged that they did not assess the potential risk factors in their daily clinical practice, before prescribing any DKR. We consider that it is easier for physicians to remember and associate the QT interval prolongation with drugs acting at a cardiovascular level. Thanks to these survey, additional information was obtained about 21.6% of the patients reported; the DKR was withdrawn to 289 of these patients, and 398 patients underwent an ECG for monitoring. Physicians valued positively the information provided, because they considered it highly useful, possibly due to the lack of time to search in bibliographic sources, and the lack of alert systems at the time of computerized prescription. A bias to be considered is that the level of acceptance by the rest of the physicians in the Area remains unknown; and maybe the professionals who answered were those more involved and committed with this type of actions conducted from the Department of Pharmacy.
It is worth highlighting that, according to the analysis subsequently conducted, the DKR was withdrawn to over half of the patients identified. However, it must be taken into account that many of the drugs withdrawn were antibiotics, with higher use in the month of the study, and with a limited time of use, something which also applies to antiemetics. Therefore, we cannot conclude that the outcomes obtained regarding the subsequent withdrawal of medication were only caused by our intervention. However, the information provided to physicians can be useful to prevent future prescriptions of these drugs to patients at risk, thus improving their safety.
Within the limitations of the study, there is the fact that patients could collect their medications from the retail pharmacy during the month analyzed, but not administer them completely (lack of therapeutic compliance), or take them simultaneously.
Another limitation is that only patients who received a NHS prescription during December, 2013 were analyzed, and the risk could not be assessed in patients in the private setting, those who did not collect their medications during the month of the study, or those who were impossible to identify because they received prescriptions by Specialized Care Physicians and/or manual prescriptions.
When conducting the analysis of interactions, medicinal plants, homeopathy, or food such as grapefruit juice were not considered. Any potential interactions with drugs obtained without prescription are also unknown. Besides, the low cooperation by physicians in returning the survey to the Department of Pharmacy has prevented us from obtaining the complete profile of risk factors, and assessing the frequency of development of adverse effects derived of the prescription of risk drugs. Therefore, it would be advisable to consider this in any future research, in order to learn about the scope and clinical relevance of these findings.
Regarding the external validity of results, we have not found many studies, and none in our country, with the same methodology and inclusion criteria, in order to establish any comparisons.
Summing up, we consider that there is a relevant prevalence detected in the Healthcare Area regarding the prescription of drugs which prolong the QT interval, taking into account that patients also had other risk factors. The Primary Care Pharmacist, through the identification of patients and their risk factors, for their subsequent report to the physicians in charge, conducts a training activity which allows a closer follow-up of this type of prescriptions, improving the quality of care and patient safety.
Conflict of Interest
The authors state that there is no conflict of interest when preparing this manuscript.
Acknowledgements
To the Primary Care Physicians in the Healthcare Area who cooperated with this study, by returning the completed questionnaire.
Communication to Congress
A. Díaz Madero, MJ. Hernández Arroyo, D. Menacho Miguel. Intervention for a safe prescription of drugs that prolong the QT interval. XIX National Congress of the Spanish Society of Primary Care Pharmacists, October, 29th-31st. Mérida.
![]() Correspondence:
Correspondence:
Correo electrónico: mjharroyo@saludcastillayleon.es
(María Jesús Hernández Arroyo).
Recibido el 18 de noviembre de 2014;
aceptado el 14 de junio de 2015.
Bibliography
1. Selzer A, Wray HW. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation. 1964;30:17-26. [ Links ]
2. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013-22. [ Links ]
3. INFAC. Medicamentos e intervalo QT; 2013 (consultado 7 Abr 2014). Disponible en: http://www.osakidetza.euskadi.net/contenidos/informacion/cevime_infac/es_cevime/adjuntos/INFAC_Vol_21_n_6_Medicamentos_intervalo_QT.pdf. [ Links ]
4. Van Noord C, Eijgelsheim M, Stricker BHC. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70:16-23. [ Links ]
5. Shah RR. Drug-induced QT interval prolongation: does ethnicity of the thorough QT study population matter? Br J Clin Pharmacol. 2013;75:347-58. [ Links ]
6. Food and Drug Administration. International conference harmonization guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs; 2005 (consultado 29 Abr 2014). Disponible en: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm129357.pdf. [ Links ]
7. Kunkler K. Acquired long QT syndrome: risk assessment, prudent prescribing and monitoring, and patient education. J Am Acad Nurse Pract. 2002;14:382-9. [ Links ]
8. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. Scientific World Journal. 2012;2012:212178. [ Links ]
9. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934-47. [ Links ]
10. Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35:361-90. [ Links ]
11. Viskin S, Justo D, Halkin A, Zeltser D. Long QT syndrome caused by noncardiac drugs. Prog Cardiovasc Dis. 2003;45:415-27. [ Links ]
12. Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J. 2000;21:1216-31. [ Links ]
13. Villamañán E, Armada E, Ruano M. Prolongación del intervalo QT inducido por fármacos: ¿conocemos sus riesgos?. Med Clin (Barc). 2014 Mar 18. pii: S0025-7753(14)00120-1. http://dx.doi.org/10.1016/j.medcli.2014.01.027. (Epub ahead of print). [ Links ]
14. Web de Arizona Center for Education and Research on Therapeutics (consultado 17 Dic 2013). Disponible en: https://www.crediblemeds.org/healthcare-providers. [ Links ]
15. Agencia Española de Medicamentos y Productos Sanitarios. Nota informativa 19/2011. Citalopram y prolongación del intervalo QT del electrocardiograma; 2011 (consultado 22 Mar 2014). Disponible en: http://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2011/NI-MUH_19-2011.htm. [ Links ]
16. Agencia Española de Medicamentos y Productos Sanitarios. Nota informativa 23/2011. Escitalopram: prolongación del intervalo QT del electrocardiograma; 2011 (consultado 22 Mar 2014). Disponible en: http://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2011/NI-MUH_23-2011.htm. [ Links ]
17. Curtis LH, Østbye T, Sendersky V, Hutchison S, Allen LaPointe NM, Al-Khatib SM, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003;114:135-41. [ Links ]
18. Allen LaPointe NM, Curtis LH, Chan KA, Kramer JM, Lafata JE, Gurwitz JH, et al. Frequency of high-risk use of QT-prolonging medications. Pharmacoepidemiol Drug Saf. 2006;15:361-8. [ Links ]
19. Baptista R, Silva S, Dias P, Monteiro P, Feio J, Providência LA. In-hospital prescription of QT-prolonging drugs in a cohort of more than 100,000 patients. Int J Cardiol. 2011;147:165-6. [ Links ]
20. Food and Drug Administration. FDA Drug Safety Communication: azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms; 2013 (consultado 5 May 2014). Disponible en: http://www.fda.gov/drugs/drugsafety/ucm341822.htm. [ Links ]
21. Food and Drug Administration. FDA Statement regarding azithromycin (Zithromax) and the risk of cardiovascular death; 2012 (consultado 5 May 2014). Disponible en: http://www.fda.gov/Drugs/DrugSafety/ucm304372.htm. [ Links ]











 texto en
texto en