Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Farmacia Hospitalaria
versión On-line ISSN 2171-8695versión impresa ISSN 1130-6343
Farm Hosp. vol.39 no.5 Toledo sep./oct. 2015
https://dx.doi.org/10.7399/fh.2015.39.5.8981
ARTÍCULO DE OPINIÓN
Vedolizumab in Crohn Disease genesis-sefh drug evaluation report*
Vedolizumab en Enfermedad de Crohn; Informe de evaluación GENESIS-SEFH
Jenifer González Chávez1, Rocío Asensi Díez1, Rocío Tamayo Bermejo1, Emilio Jesús Alegre del Rey2 and Noemí Martínez López de Castro3
1Hospital Regional Universitario de Málaga, Málaga.
2Hospital Universitario de Puerto Real, Cádiz.
3Hospital de Meixoeiro, Vigo. Spain.
*This is a summary of the most relevant references; the full list can be retrieved from the original drug evaluation report GENESIS-SEFH (Spanish): (http://gruposdetrabajo.sefh.es/genesis/).
*This paper is an abstract of vedolizumab drug evaluation report by GENESIS-SEFH (Group for Innovation, Assessment, Standardisation and Research in the Selection of Drugs of the Spanish Society of Hospital Pharmacy) that can be retrieved in his entire form from GENESIS web (http://gruposdetrabajo.sefh.es/genesis/). This evaluation has been made with the aid of MADRE 4.0 application1.
Introduction
Crohn's Disease (CD) is a chronic and incurable process. The incidence of new CD cases presents a high variation between geographical areas. In Spain, data published in 2014 have shown an incidence of 8.9 cases per 100,000 inhabitants and year. The prevalence in Spain, though very difficult to determine, has been estimated about 137.17 cases / 100,000 inhabitants (95% confidence interval 114-160)2.
The objective of treatment is to achieve and to sustain complete remission of the disease, and to prevent and to treat complications. The clinical heterogeneity of CD will require individualized treatment, which depends on multiple factors, including localization, severity, evolution pattern, previous response to treatment, and presence of complications.
Overall, CD patients have recurrent attacks, with acute exacerbations interspersed with periods of remission or less active disease. Treatment is largely directed at symptom relief rather than cure, and active treatment of acute disease (inducing remission) should be distinguished from preventing relapse (maintaining remission)3.
Glucocorticoids appear as first line of treatment. For patients who cannot tolerate or are contraindicated glucocorticoids, oral budesonide can be considered. Aminosalicylates such as aminosalicylic acid or mesalazine (5-ASA) are another alternative to corticoids. Treatment with budesonide or 5-ASA cannot be used in case of severe exacerbations of the disease.
Azathioprine (AZA) or 6-mercaptopurine (6-MP) must be added to treatment if there are 2 or more exacerbations within one year, or if there has been low response to glucocorticoids. If AZA or 6-MP cannot be tolerated, or if there is a deficiency of thiopurine methyltransferase, treatment can be combined with glucocorticoids and methotrexate.
In case of no response or contraindication to conventional therapy, biologic therapy with anti-tumour necrosis factor alpha (TNF-α) drugs is recommended for severe exacerbations of the disease. These drugs present two important characteristics: fast onset of action for induction of clinical remission, and efficacy in maintenance of the remission induced by the drug itself. There are two anti-TNF drugs approved in Spain for CD: infliximab (IFX) and adalimumab (ADA).
The current recommendation would be to use conventional sequential treatment, with well established periods of time based on the evolution of the disease, in order to introduce the biological-immunomodulator treatment without delay; this has been called an "accelerated step-up" strategy4,5.
A meta-analysis made by Peyrin-Biroulet et al6 has shown that IFX, ADA and certolizumab are effective drugs for induction of remission. Certolizumab has only been approved for CD in Switzerland, but not in the rest of Europe. Certolizumab, as well as natalizumab, have been approved by the FDA for induction of remission in CD7,8.
There are no comparative "head to head" clinical trials (CTs) between the two biological agents available in our country (IFX and ADA), in order to guide the choice5.
If the anti-TNF drug loses efficacy after one year, the administration interval can be shortened, or the dose can be increased. If regardless of this, there is no increase in efficacy, treatment can be continued with a second anti-TNF4,5,9,10.
Vedolizumab (VDZ) (Entyvio®, Lab Takeda Pharma) appears in this context; it has been approved by the European Medicines Agency (EMA)11 and the Spanish Agency of Medicines and Healthcare Products (AEMPS)12 for treatment of adult patients with moderately to severely ulcerative colitis and active Crohn's disease who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a TNFα antagonist.
VDZ is a humanized immunoglobulin G1 (IgG1) monoclonal antibody directed against the human lymphocyte integrin a4|37. The a4|37 integrin is expressed on the surfaces of both T and B lymphocyte subpopulations, including at the surface of a discrete subset of memory T lymphocytes that preferentially migrate into the gastrointestinal tract. The recommended dose regimen is 300 mg administered by intravenous infusion at zero, two and six weeks and then every eight weeks thereafter.
Efficacy
Two CTs have been carried out with VDZ in CD: GEMINI II13 which compared VDZ vs. placebo for treatment of moderate-severe CD, and GEMINI III14 which compared VDZ vs. placebo for treatment of CD in patients with previous TNF antagonist failure.
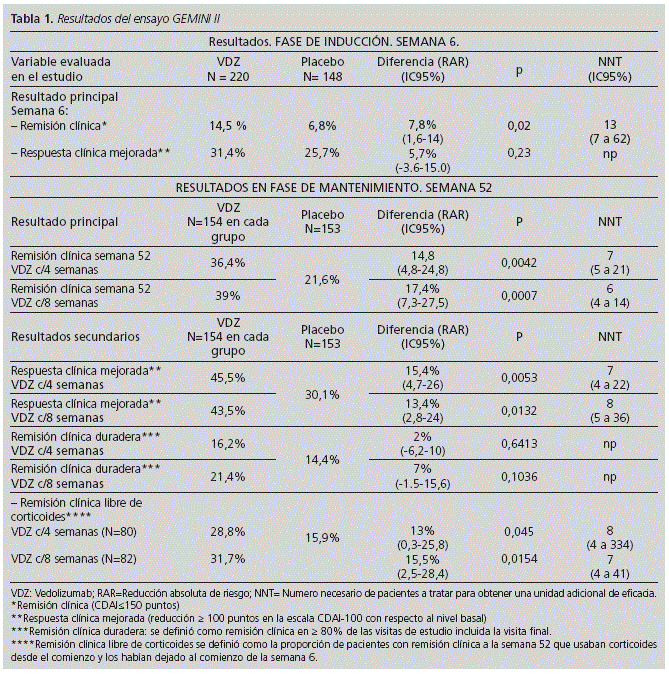
GEMINI II is an international Phase III, randomized, parallel-group, double blind, placebo controlled study consisted of separate induction and maintenance trials which included 1,115 patients between both trials. It consists of two phases: an induction phase and a maintenance phase. In this clinical trial, approximately 50% of patients had had treatment failure with one or more TNF antagonists. The aim of this study was to determine the effect of VDZ in patients who have failed 1 or more standard therapies for CD, including immunomodulators (AZA, 6-MP, or methotrexate) and TNFαantagonists. To ensure that the efficacy of VDZ could be evaluated in patients who are naive to TNFαantagonists, enrollment of patients with previous TNFαantagonist exposure was to be limited to no more than 50% of the overall study population.
In the double-blind induction trial (cohort 1), patients were randomly assigned, in a 3:2 ratio, to receive intravenous VDZ, at a dose of 300 mg, or placebo at weeks 0 and 2 and were followed through week 6, at which time disease status was assessed.
To fulfill the sample-size requirements for the maintenance trial, additional patients were enrolled in an open-label group (cohort 2), which received the same VDZ induction regimen that was used for the patients assigned to VDZ in cohort 1. Patients from both cohorts who had a clinical response (i.e., >70-point decrease in the CDAI [Crohn's Disease Activity Index] score) with VDZ at week 6 were randomly assigned, in a 1:1:1 ratio, to continue in a blinded fashion to receive VDZ every 8 weeks, VDZ every 4 weeks, or placebo, for up to 52 weeks.
The two primary end points in the trial of induction therapy were clinical remission (CDAI score of <150 points and CDAI-100 response (>100-point decrease in the CDAI score) at week 6.
CDAI is the gold standard used in clinical trials in order to quantify clinical activity. From a clinical point of view, the disease is classified into: mild activity (150-220 CDAI), moderate activity (220-450 CDAI) and severe activity (>450 CDAI).
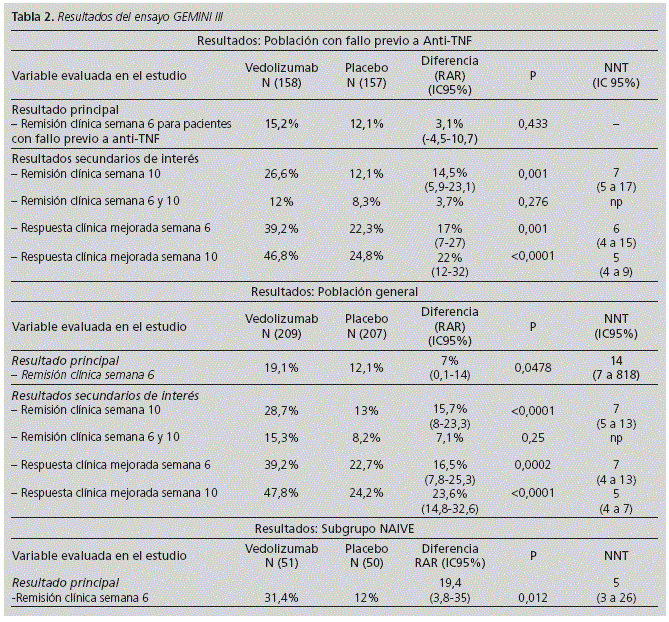
GEMINI III is an international Phase III, randomized, placebo-controlled, double-blind, multinational, multicenter trial which included 416 patients. The primary objective of this study was to determine the effect of VDZ induction therapy on clinical remission at week 6 in patients with CD and previous TNF antagonist failure (75% of enrolled patients). Eligible patients then were assigned randomly 1:1 to receive VDZ 300 mg or placebo, administered intravenously at weeks 0, 2, and 6. Secondary objectives included determining the effects of VDZ on the CDAI-100 response at week 6 and clinical remission at week 10 in the TNF antagonist-failure population and on remission at weeks 6 and 10 in the overall population.
Tables 1 and 2 show the main results of these trials: GEMINI II y III respectively.
Safety
Based on the clinical trial experience, the most frequent adverse events were: nasopharyngitis, headache, arthralgia, abdominal pain and infections; and the most severe were: anaemia, disease exacerbation, anal and abdominal abscesses, severe infections, and various neoplasias.
In GEMINI II, a female patient developed breast cancer during the induction phase; while in the maintenance phase, there was a case of carcinoid tumour in the appendix, another case of squamous cell carcinoma, and a case of basal cell carcinoma.
In GEMINI III, a male patient developed neurological symptoms during the clinical trial, and even though Progressive Multifocal Leukoencephalopathy (PML) was ruled out, the patient was withdrawn from the study because he presented an ependimoma, which was the only neoplasia developed during this clinical trial.
There have been no reports of extra-pulmonary or systemic tuberculosis with VDZ.
The safety and efficacy of vedolizumab in children aged 0 to 17 years old have not been established. No data are available. No interaction studies have been performed. It is recommended to continue studying its long-term safety.
Economic area
Because the price of VDZ has not yet been established in Spain, the economic analysis has been conducted with the invoicing price taken from the application of medications in special situations15.
With this reservation, and according to incremental cost analysis, VDZ appears as the most expensive alternative, compared vs. anti-TNFs: ADA and IFX.
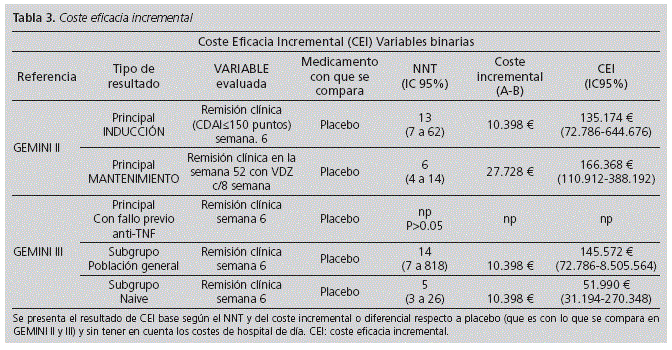
According to the GEMINI II data and the incremental cost of treatment vs placebo (regardless administration costs in hospital), the estimated additional cost for each additional patient who achieves clinical remission at Week 6 would be € 135,173. According to the same study, the estimated additional cost for each additional patient with durable clinical remission at Week 52 with VDZ every eight weeks would be € 166,368.
According to the GEMINI III data and the incremental cost of treatment vs placebo (regardless administration costs in hospital), the estimated additional cost for each additional patient in the "overall study population" (which included: prior TNF antagonists failure and TNF antagonists-naive patients) who achieve clinical remission at week 6 would be € 145,572. According to the same study, the estimated additional cost for each patient in the "naive" sub-group who achieve clinical remission at Week 6 would be € 51,990. (Table 3).
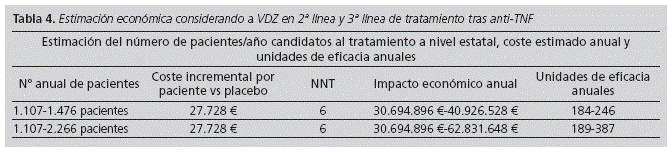
In terms of the budget impact at national level, if we consider VDZ as second line, according to the failure rates obtained from CTs: GEMINI II and III, its budget impact after failure to anti-TNF would be € 30,694,896-€ 40,926,528 per year in order to treat 1,107-1,476 patients in Spain.
In third line treatment, its budget impact after failure to two anti-TNF, where currently there is not therapeutic option, would be € 30,694,896 € to € 62,831,648 € per year in order to treat 1,107-2,266 patients in Spain. (Table 4).
Additional considerations
Placebo was used as comparator in both clinical trials, and VDZ showed superiority vs. placebo in both studies. However, there are currently two anti-TNF drugs approved in Spain and used in clinical practice for moderate-severe CD treatment after failure with conventional therapy; namely, IFX and ADA. These drugs have demonstrated their efficacy for CD, with patients achieving fast remission. However, both drugs will cease being equally effective over time, and it is necessary to change their dosing regimen or the type of anti-TNF drug in order to control the disease outbreaks.
Both, GEMINI II and GEMINI III included TNF antagonist-failure population: approximately 50% and 75% in GEMINI II and III respectively. However, the different rates of failure to one or two anti-TNFs in both clinical trials appear to be striking. The population with failure to one anti-TNF drug was 21% and 22%-28% (GEMINI II and III respectively), and with failure to two anti-TNFs was 21% and 39-43% (GEMINI II and III respectively).
In GEMINI II, it was observed that VDZ is effective for the induction of clinical remission, defined as CDAI< 150 scores at week 6; but not in terms of CDAI-100 response at week 6.
Among patients who achieved clinical remission with VDZ at week 6, the best response rates: clinical remission and clinical remission in glucocorticoid-free patients were in those patients receiving VDZ every 8 weeks. CDAI-100 responses were better in patients receiving VDZ more frequently (every 4 weeks).
In the induction phase, we observed that clinical remission and CDAI-100 response were better for those patients who continued treatment with glucocorticoid than those who were treated with VDZ alone until week 6.
In GEMINI III, for the primary outcome, proportion of patients in clinical remission at week 6 for the TNF antagonist-failure population, no statistically significant difference was observed between the VDZ and placebo groups. However, in the overall population, a greater proportion of VDZ-treated patients than placebo-treated patients were in clinical remission at week 6
The CDAI-100 response at week 6 for the TNF antagonist-failure population and overall population was statistically significant in patients treated with VDZ. Effects of VDZ on clinical remission may not become evident until between weeks 6 and 10. It could be deduced that the time to achieve remission with VDZ may be ten weeks in patients with CD, particularly in patients with previous TNF antagonist failure. Results in the TNF antagonist-failure population showed a clinically important increase over time in the proportion of VDZ-treated patients in remission, from 15.2% at week 6 to 26.6% at week 10. However, the remission rate in placebo-treated patients remained constant at 12.1% at weeks 6 and 10.
If we analyze the results of clinical remission at week 10, the difference in ARR between TNF antagonist-failure population and the overall population was small (14.5% vs. 15.7% respectively). This result could suggest the need of an additional treatment dose (weeks 0, 2 and 6), and a longer time (10 weeks) of follow-up to observe a clinically relevant benefit.
Clinical remission at weeks 6 and 10 was not achieved either in TNF antagonist-failure population and the overall population, but better results in this outcome were achieved in the overall population.
In the TNF antagonist-naive population the clinical remission at week 6 was 31.4% vs. 12% (VDZ vs. placebo).
In both clinical trials best results were observed with the simultaneous use of glucocorticoids.
The lack of statistical significance of primary outcome results contrasts with the GEMINI II induction study results in patients with previous TNF antagonist failure. However, several patient characteristics and design parameters differed between these 2 studies (e.g., differences in upper CDAI score cut-off values, defined by entry criteria, and in mean CDAI scores, and re-randomization at week 6 in GEMINI II). Observed differences in week 6 remission rates between overall populations of the two studies may be attributable to variations between 2 otherwise similar patient populations, including proportions of patients with previous exposure to 1, 2, or 3 TNF antagonists (GEMINI II, 47.6%; GEMINI III, 75.7%). The upper bound of patients' CDAI scores (GEMINI II, 450; GEMINI III, 400) or random variation could have accounted for the observed differences in subgroup analyses of week 6 remission rates among TNF antagonist-naive patients.
In conclusion, effects of VDZ induction therapy were modest overall, according to the results observed in both CTs. The maintenance effects were not evaluated in GEMINI III. However, the modest efficacy of VDZ induction therapy was contrasted by the benefit of VDZ maintenance therapy over the course of 52 weeks in GEMINI II. In this study, in those patients with clinical remission in the induction phase, clinical remission occurred in 39% and 36.4% of those who continued with VDZ every 8 and every 4 weeks, respectively vs. 21.6% of patients on placebo arm. Effects were similar between clinical remission at week 52 and clinical remission of glucocorticoids-free population.
Conclusion; therapeutic positioning and conditions of use
In view of the efficacy and safety results, the proposed positioning is considered Category D-1 (category explanation is included in MADRE program1): It is Included in the formulary with specific recommendations: as an alternative in the second or third line of treatment for moderate to severe Crohn's Disease.
![]() Correspondence:
Correspondence:
Correo electrónico: rocio.asensi.sspa@juntadeandalucia.es
(Rocío Asensi Díez).
Bibliography
1. Marin R, Puigventos F, Fraga MD, Ortega A, Lopez-Briz E, Arocas V, et al. Group for Innovation, Assessment, Standardisation and Research in the Selection of Drugs (GENESIS) of the Spanish Society of Hospital Pharmacy (SEFH). Support method for decision making in assessment and appraisal of medicines (MADRE). Version 4.0. Madrid: SEFH (ed.), 2013. ISBN: 978-84-695-76298. Available at http://gruposdetrabajo.sefh.es/genesis/genesis/Documents/MADRE 4_0_Procedures manual_Dec_2013.pdf. [ Links ]
2. Lucendo AJ, Hervías D, Roncero Ó, Lorente R, Bouhmidi A, Angueira T, et al. Epidemiology and temporal trends (2000-2012) of inflammatory bowel disease in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol. 2014 Dec; 26(12):1399-407. [ Links ]
3. NICE clinical guideline 152. Crohn's disease. Management in adults, children and young people. October 2012. (Acceso el 25/08/2014). Disponible en: http://www.nice.org.uk/guidance/cg152. [ Links ]
4. Cabriada JL, Vera I, Domènech E, Barreiro-de Acosta M, Esteve M, Gisbert JP, et al. (Grupo GETECCU). Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa sobre el uso de fármacos antifactor de necrosis tumoral en la enfermedad inflamatoria intestinal (2013). Gastroenterol Hepatol. 2013; 36(3):127-146. [ Links ]
5. Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. European Crohn's and Colitis Organisation (ECCO). The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Current management. Journal of Crohn's and Colitis. 2010; 4(1): 28-62. [ Links ]
6. Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel J-F. Efficacy and safety of anti-tumor necrosis factor agents in Crohn's disease: a meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol 2008; 6 (6):644-53. [ Links ]
7. Ficha técnica Cimzia®. U.S. Food and Drug Administration (FDA). (Acceso el 25/08/2014). Disponible en: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125160s215lbl.pdf. [ Links ]
8. Ficha técnica Tysabri® U.S. Food and Drug Administration (FDA). (Acceso el 25/08/2014). Disponible en: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125104s840s847s889lbl.pdf. [ Links ]
9. NICE technology appraisal guidance 187. Infliximab (review) and adalimumab for the treatment of Crohn's disease. May 2010. (Acceso el 25/08/2014). Disponible en: http://www.nice.org.uk/guidance/ta187. [ Links ]
10. Informe técnico sobre anti TNF en enfermedad inflamatoria intestinal: Infliximab y Adalimumab. SESCAM. Servicio de Salud de Castilla-La Mancha. (Acceso el 25/08/2014). Disponible en: http://sescam.castillalamancha.es/sites/sescam.castillalamancha.es/files/documentos/farmacia/infliximab_y_adalimumab.pdf. [ Links ]
11. EMA. Entyvio. Assesment report. EMA/CHMP/676643/2013. Committee for Medicinal Products for Human Use (CHMP). 20 March 2014. (Acceso el 22/07/2014). Disponible en: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Public_assessment_report/human/002782/WC500168530.pdf. [ Links ]
12. Ficha técnica Entyvio®. European Public Asessment Report. (Acceso el 25/08/2014). Disponible en: http://www.ema.europa.eu/docs/es_ES/document_library/EPAR__Product_Information/human/002782/WC500168528.pdf. [ Links ]
13. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2013; 369:711-21. [ Links ]
14. Sands BE, Feagan BG, Rutgeerts P, Colombel J-F, Sandborn WJ, Sy R, et al. Effects of Vedolizumab Induction Therapy for Patients with Crohn's Disease in whom Tumor Necrosis Factor Antagonist Treatment Failed. Gastroenterology 2014; 147:618-627. [ Links ]
15. Gestión de medicamentos en situaciones especiales de la Agencia Española de Medicamentos y Productos Sanitarios. Ministerio de Sanidad, Servicios Sociales e Igualdad. (Acceso el 07/03/2015). Disponible en: https://mse.aemps.es/mse/loginForm.do. [ Links ]











 texto en
texto en