Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Farmacia Hospitalaria
versão On-line ISSN 2171-8695versão impressa ISSN 1130-6343
Farm Hosp. vol.40 no.1 Toledo Jan./Fev. 2016
https://dx.doi.org/10.7399/fh.2016.40.1.8918
ORIGINALES
Chemotherapy near the end of life; assessment of the clinical practise in onco-hematological in adult patients
Quimioterapia al final de la vida; análisis de la práctica clínica en pacientes adultos onco-hematológicos con cáncer
Pilar Taberner Bonastre1, María Teresa Taberner Bonastre2, Enrique Soler Company1 and María Dolores Pérez-Serrano Lainosa1
1Hospital Arnau de Vilanova, Valencia.
2Hospital Universitario de la Ribera, Alzira. Spain.
ABSTRACT
Objective: ensure a good quality of life in the last phase of onco-hematological patients should be the primary goal, despite this, we have little data at European level and published studies are contradictory. Nevertheless, most of them agree saying that administrating chemotherapy near the end of life impacts negatively in the patients quality of life. The main objective of this study is to analyze the treatment non-aggressiveness parameters in onco-hematological patients. The secondary objective is to do a describing study of the clinical variables of the patients who receive chemotherapy at the end of life and the treatments more used.
Methods: a retrospective observational study was conducted in a tertiary hospital. Both, oncological and hematological patients receiving chemotherapy (oral or intravenous) between January and December 2013 who were receiving chemotherapy in the last 90 days before death, were included.
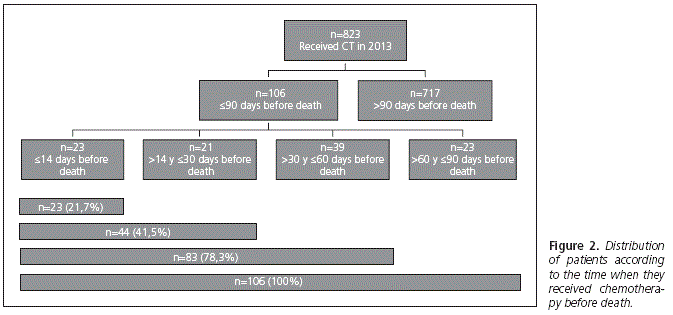
Results: there were 823 patients that were threated between January and December of 2013. Of these 106 (13%) met the inclusion criteria to be analyzed. There were a 14.1% (n = 93) of oncological and a 8.4% (n = 13) of hematologic patients that maintained the antineoplasic treatment during the last three months before death. A 21.7% (n = 23) of the patients received chemotherapy in the last two weeks of life, 41.5% (n = 44) in the last 30 days and 78.3% (n = 83) in the last two months of life. There was a 67.9% (n = 72) of patients that had hospital admissions during their last three months of life, 47,2% (n = 50) during the last month, 33% (n = 35) during the last two weeks and 10,4% (n = 11) during the last three days of life. A 25,5% (n = 27) of patients had more than one hospital admission during their last 90 days.
Conclusions: according to the Earle et al. criteria, our population had been treated aggressively. We need more scientific evidence with consolidate date that allows us to establish a unified criteria for the selection of patients with advanced cancer who may benefit from receiving antineoplasic treatments.
Key words: Chemotherapy; Neoplasm; Death; Hospitalization.
RESUMEN
Objetivo: garantizar una buena calidad de vida en la última fase de los pacientes onco-hematológicos debería ser nuestro principal objetivo; a pesar de ello, disponemos de pocos datos a nivel europeo y los estudios publicados son contradictorios. No obstante, la mayoría coinciden en que administrar quimioterapia en la etapa final de la vida impacta de forma negativa en la calidad de vida del paciente. El objetivo principal del estudio es analizar los indicadores de no agresividad del tratamiento en pacientes onco-hematológicos. Como objetivo secundario, realizar un estudio descriptivo de las variables clínicas de los pacientes a los que se les administra quimioterapia en la fase final de la vida y los esquemas más utilizados.
Método: se realizó un estudio observacional retrospectivo en un hospital de tercer nivel. Se incluyeron todos los pacientes oncológicos y hematológicos que recibieron quimioterapia (oral o intravenosa) entre enero y diciembre de 2013, a los que se les administró la última quimioterapia en los 90 días previos al fallecimiento.
Resultados: se analizaron 823 pacientes que recibieron tratamiento entre enero y diciembre de 2013. De ellos, 106 (13%) cumplían los criterios de inclusión. Un 14,1% (n = 93) de los pacientes oncológicos y un 8,4% (n = 13) de los hematológicos que habían recibido quimioterapia durante el último año de vida lo seguían haciendo en los últimos tres meses. Un 21,7% (n = 23) de los pacientes recibieron quimioterapia en las dos últimas semanas, 41,5% (n = 44) en los últimos 30 días y 78,3% (n = 83) en los últimos dos meses de vida. El 67,9% (n = 72) de los pacientes ingresaron en el hospital durante los últimos tres meses de vida, el 47,2% (n = 50) lo hicieron durante el último mes, el 33% (n = 35) durante las dos últimas semanas y el 10,4% (n = 11) durante los últimos tres días de vida. El 25,5% (n = 27) de los pacientes ingresaron en más de una ocasión durante los 90 días previos al fallecimiento.
Conclusiones: según los criterios establecidos por Earle et al, la población del estudio se trató de manera agresiva al final de la vida. Es necesario disponer de una evidencia científica, que todavía en la actualidad carece de una base consolidada, con la que establecer criterios para la selección de pacientes con cáncer en estadios avanzados que puedan beneficiarse de recibir tratamientos antineoplásicos.
Palabras clave: Quimioterapia; Neoplasia; Muerte; Hospitalización.
Contribution to scientific literature
Quality of life in oncology patients is still one of the essential cornerstones of research in the treatment of onco-haematological patients. Despite this, there is currently little information available at European level regarding quality of life at the last stage of the disease in patients with cancer. This study analyzes some intensity parameters for antineoplastic treatments at the end of patients' lives. This analysis includes both intravenous and oral antineoplastic treatments. The inclusion of oral administration treatments represents a differentiating point from the majority of studies conducted so far, which were focused on intravenous treatments. The outcomes from our study demonstrate that the majority of patients in our population are receiving an aggressive treatment which is far from achieving the main objective of improving patients' quality of life.
Though the outcomes obtained in this study cannot be applied to other patient populations, the data presented here show the need of a higher involvement by the healthcare team regarding therapeutic decision making. To this end, it is necessary to have scientific evidence available, which currently still lacks a well-established basis, in order to determine the criteria for selecting patients with cancer in advanced stages who might benefit from receiving antineoplastic treatments. The first step in order to achieve this will be to overthrow the ill-conceived idea that antineoplastic treatments will help, under any circumstances, to improve the quality of life of patients with cancer in advanced stages.
Introduction
During the past decades, the launch of new antineoplastic agents has increased the therapeutic options and life expectancy of patients with neoplasia. Treatments with oral administration have acquired special relevance, both because they represent a therapeutic option, and due to the simplification in administration. Despite these advances, tumours are currently the first cause of death in men, the second in women, and are responsible for 27.5% of deaths in Spain, according to data published in January, 2014 by the Instituto Nacional de Estadística (National Statistics Institute)1.
Around 70% of patients with disseminated disease are receiving chemotherapy with palliative intent2. The decision to initiate chemotherapy depends mainly on patient's status3, disease stage4, and chemosensitivity5 of the tumour. Treatment interruption in the final stages of life is considered a factor determining good clinical practice6,7. This is due, among other causes, to the reduction in the impact on overall survival after various lines of treatment in patients on advanced stages8,9, the development of adverse effects derived of treatment, and the cost for the healthcare system.
To ensure a good quality of life in the last stage of onco-haematological patients should be the main objective and, despite this, we have little data at European level5,8,10, those studies published are contradictory, and the majority coincides that administering chemotherapy during the final stage of life will have a negative impact on patients' quality of life9.
Studies published so far have used as quality of life indicators the number of hospitalizations and treatments received during the weeks before patients' death5,11. Earle et al.7 defines the concept of treatment non-aggressiveness as chemotherapy administration below 10% during the last 14 days of life, and below 4% of patients admitted to hospital on more than one occasion during the month before death.
Other studies12-15 have used criteria such as admission to Intensive Care Units, visits to ER, hospitalizations, or inclusion in Phase I-II clinical trials.
The main objective of this study is to analyze the indicators of treatment non-aggressiveness in onco-haematological patients. The secondary objective is to conduct a descriptive study of clinical variables in patients who are administered chemotherapy during their final stage of life, and the most widely used regimens.
Materials and methods
A retrospective observational study was conducted in a third level hospital, covering a population of approximately 310,000 inhabitants. The study included all oncologic and haematologic patients who received chemotherapy (oral or intravenous) between January and December, 2013, and who had their last chemotherapy administered in the final stage of their lives. For the purpose of analysis, the period of time considered was the 90 days previous to death. Follow-up of those patients included was continued until March, 2014.
Data collection was conducted with the computer programs used in the Hospital Pharmacy for onco-haematological patient care: Farmasyst® and Farmis-Oncofarm®, and the computerized clinical record programs OrionClinic® and Abucasis®. The variables collected were: gender, age, prescribing hospital unit, diagnosis, disease stage, performance status, date of the latest hospitalizations, and cause of death. The variables collected associated with treatment included: time since the last administration until death, lines of treatment received, and drugs included in the last treatment administered.
With the objective of assessing the functional status of the patient, the score in the Eastern Cooperative Oncologic Group (ECOG) Scale was collected, as entered in the clinical record on the day of the last chemotherapy administered before death. The number of hospital admissions within the last three months was also assessed, and the time elapsed since the last hospital admission until patient's death.
For descriptive analysis, the statistical program Stata® version 12.1 was used.
Outcomes
In 2013, 2,758 patients were managed in the Oncology Department, and 949 in the Haematology Department. Out of them, 24.3% (n = 669) and 16.2% (n = 154), respectively, received chemotherapy. Therefore, 823 patients who received treatment between January and December, 2013, were analyzed. Out of these, 106 (13%) met the inclusion criteria.
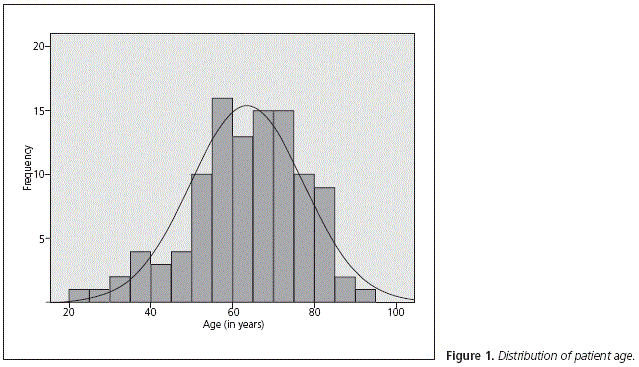
The median age of patients included was 64.6 years (range 21.9-92.5) (Fig. 1); 68.9% (n = 73) were male and 31.1% (n = 33) were female.
The hospital unit of origin of said patients was Haematology for 12.3% (n = 13) and Oncology for 87.7% of cases (n = 93).
Both intravenous and oral administration drugs were included in the treatment. A 14.1% (n = 93) of oncological patients and 8.4% (n = 13) of haematological patients who had received chemotherapy during their last year of life continued receiving it during their last three months.
The distribution of patients who received antineoplastic drugs in their last 90 days of life appears in figure 2.
Data regarding ECOG scale scores at treatment initiation was available in 87.7% of those patients analyzed. The majority (67% n = 71) presented a 0-1 score in the ECOG scale; 18.9% (n = 20) presented a score of 2, and a minority (1.9%, n = 2) had ECOG 3.
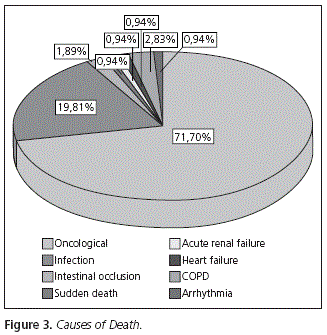
The majority of patients (77.6%, n = 76) presented Stage IV disease at the time of their death, which was secondary to their basal disease. A 28.3% of patients (n = 30) presented complications secondary to treatment as cause of death (Fig. 3).
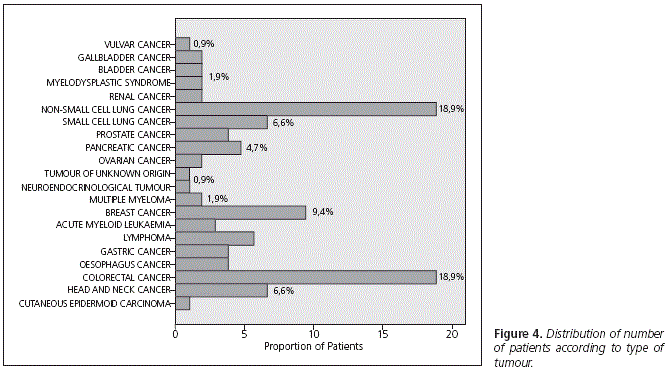
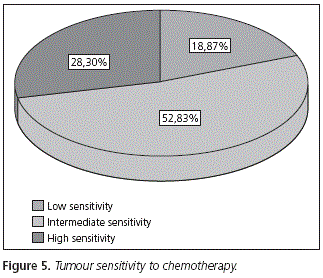
The distribution of the tumours suffered by patients included in the study appears in figure 4. The highest percentage of tumours had intermediate sensitivity to treatment, according to the classification by Martoni et al.5 (Fig. 5)
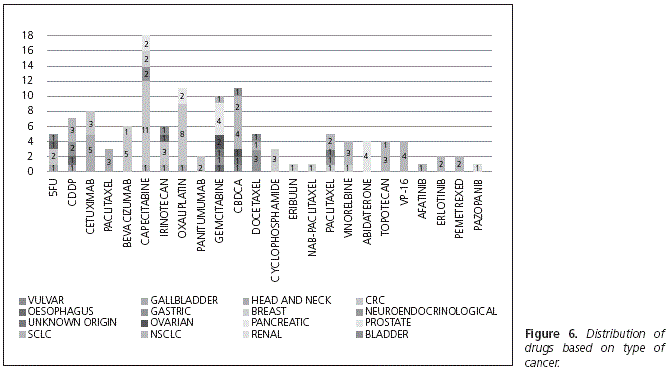
The most widely used therapeutic regimen was capecitabine as monotherapy, administered in 6 patients, as well as the capecitabine + oxaliplatin combination. Topotecan was administered to 5 patients. The following treatments were received by 4 patients each: abiraterone, docetaxel, gemcitabine, azacitidine, cetuximab, doxorubicin, erlotinib, as well as the following combinations: bevacizumab + capecitabine + oxaliplatin; carboplatin + etoposide; carboplatin + paclitaxel, and cetuximab + paclitaxel. Capecitabine was the drug received by the highest number of patients: 18.9% (n = 20), followed by oxaliplatin: 12.2% (n = 13) and gemcitabine: 9.4% (n = 10) (Fig. 6).
The median number of treatment regimens received was 2 (range 1-7). There were 10 patients who received more than 3 different regimens.
A 67.9% (n = 72) of patients were admitted to hospital during their last three months of life; 47.2% (n = 50) during their last month; 33% (n = 35) during their last two weeks, and 10.4% (n = 11) during their last 3 days of life. A 25.5% (n = 27) of patients were admitted in more than one occasion throughout the 90 days previous to their death.
Discussion
The use of chemotherapy during the last stages of life of cancer patients is a controversial issue, which Emanuel et al.11 dealt with over a decade ago, and that continues being studied nowadays. The outcomes of our study show that the proportion of patients in the Oncology Department who receive chemotherapy at the last stage of their lives is similar to the one by Earle et al.13.
Regarding the use of chemotherapy during the final stages of life, relative to the total number of patients who are treated with this type of drugs, 5.8% received chemotherapy during their last month of life. This value is close to the 6.2% published by Wright et al.16, but appears far from the 22.7% published by Angelo et al.5.
The latest studies published about the use of chemotherapy in the final stage of life9 and of the disease, demonstrate this is damaging for the quality of life of patients receiving it, regardless of their performance status at treatment initiation. Adverse reactions derived of treatment can be an additional factor for a worsening in the quality of life of patients with antineoplastic treatment. The prescription to the patients in the study of capecitabine instead of 5-fluorouracil was probably considered with the aim of minimizing the development of adverse reactions17,18.
Regarding hospitalizations at the end of life, our patients were admitted in a higher proportion than those in other studies19,20. According to the criteria by Earle et al.7, our patients received aggressive treatment at the end of their lives. In order to improve clinical practice and decision making regarding chemotherapy administration at the end of life, it is considered necessary to conduct further studies to define the criteria for establishing the conditions that ensure an improvement in quality of life for patients with advanced stage cancer.
The inclusion of treatments with oral administration represents a differentiating point from the majority of studies conducted so far, which are focused on intravenous treatments. The outcomes obtained in our study demonstrate that the majority of patients in our population are receiving an aggressive treatment which is far from achieving the primary goal of improving the quality of life of patients.
A higher involvement by the healthcare team in therapeutic decision making is required. For this aim, it is necessary to have scientific evidence, which currently still lacks a solid basis21, in order to determine the criteria for the selection of patients with advanced stage cancer who might benefit from receiving antineoplastic treatments. The first step in order to achieve this will be to overthrow the ill-conceived idea that antineoplastic treatments, under any circumstances, will help to improve the quality of life of patients with advanced stage cancer9.
The limitations of this study are that this is a retrospective study which analyzed the sample in a single centre during one year, and therefore it cannot be applied to other populations.
Bibliography
1. INE: Defunciones según la causa de muerte (Internet). Madrid: Instituto Nacional de Estadística. (Actualizado 31-01-2014; citado 7-08-2014). Disponible en: http://www.ine.es/prensa/np830.pdf. [ Links ]
2. Kim MK, Lee JL, Hyun MS, Do YR, Song HS, Kim JG, et al. Palliative chemotherapy preferences and factors that influence patient choice in incurable advanced cancer. Jpn J Clin Oncol. 2008;38:64-70. [ Links ]
3. Schnipper LE, Smith TJ, Raghavan D, Blayney DW, Ganz PA, Mulvey TM, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30:1715-24. [ Links ]
4. NCI: Cáncer de seno (mama): Tratamiento para profesionales de salud (Internet). EE.UU: Instituto Nacional del Cáncer. (Revisada 08-2014, citado: 15-09-2014). Disponible en: http://www.cancer.gov/espanol/pdq/tratamiento/seno/HealthProfessional. [ Links ]
5. Angelo Martoni A, Tanneberger S, Mutri V. Cancer chemotherapy near the end of life: the time has come to set guidelines for its appropriate use. Tumori. 2007;93:417-22. [ Links ]
6. Zhang B, Nilsson ME, Prigerson HG. Factors important to patients' quality of life at the end of life. Arch Intern Med. 2012;172:1133-42. [ Links ]
7. Earle CC, Neville BA, Landrum MB, Souza JM, Weeks JC, Block SD, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17:505-9. [ Links ]
8. Adam H, Hug S, Bosshard G. Chemotherapy near the end of life: a retrospective single-centre analysis of patients' charts. BMC Palliat Care. 2014,22;13-26. [ Links ]
9. Prigerson HG, Bao Y, Shah MA, Paulk ME, LeBlanc TW, Schneider BJ, et al. Chemotherapy use, performance status, and quality of life near death chemotherapy at the end of life. JAMA Oncol. (revista en Internet). 2015 (citado 20/8/2015). Disponible en: http://oncology.jamanetwork.com/article.aspx?articleid=2398177. [ Links ]
10. Näppä U, Lindqvist O, Rasmussen BH, Axelsson B. Palliative chemotherapy during the last month of life. Ann Oncol. 2011;22:2375-80. [ Links ]
11. Emanuel EJ, Young-Xu Y, Levinsky NG, Gazelle G, Saynina O, Ash AS. Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med. 2003;138:639-43. [ Links ]
12. Kao S, Shafiq J, Vardy J, Adams D. Use of chemotherapy at the end of life in oncology patients. Ann Oncol. 2009;20:1555-59. [ Links ]
13. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiviness of cancer care near the end of life. J Clin Oncol. 2004;22:315-21. [ Links ]
14. Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block SD. Identifying potential indicators of the quality of en-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133-38. [ Links ]
15. Enzinger AC, Zhang B, Weeks JC, Prigerson HG. Clinical trial participation as part of end-of-life cancer care: associations with medical care and quality of life near death. J Pain Symptom Manage. 2013;47:1078-90. [ Links ]
16. Wright AA, Zhang B, Keating NL, Weeks JC, Prigerson HG. Associations between palliative chemotherapy and adult cancer pantients' end of life care and place of death: prospective cohort study. Br Med J. 2014;348:g1219. [ Links ]
17. Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27:23-44. [ Links ]
18. Hoff P.M, Ansari R, Batist G., et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: Results of a randomized phase III study. J Clin Oncol. 2001;19:2282-92. [ Links ]
19. Zerillo JA, Stuver SO, Fraile B, Dodek AD, Jacobson JO. Understanding oral chemotherapy prescribing patterns at the end of life at a comprehensive cancer center: analysis of a Massachusetts payer claims database. JOP (revista en Internet). 2015 (citado 22/8/2015). Disponible en: http://jop.ascopubs.org/content/early/2015/08/04/JOP.2015.003921.abstract. [ Links ]
20. Keam B, Oh Dy, Lee SH, Kim DW, Kim MR, Im SA, et al. Aggressiveness of cancer-care near the end-of-life in Korea. Jpn J Clin Oncol. 2008;38:381-6. [ Links ]
21. Hong JH, Rho SY, Hong YS. Trends in the aggressiveness of end-of-life cancer for advanced stomach cancer patients. Cancer Res Treat. 2013;45:270-75. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: pitabo@hotmail.com
(Pilar Taberner Bonastre).
Recibido: el 7 de marzo de 2015;
Aceptado: el 2 de octubre de 2015.











 texto em
texto em