Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Farmacia Hospitalaria
versión On-line ISSN 2171-8695versión impresa ISSN 1130-6343
Farm Hosp. vol.40 no.2 Toledo mar./abr. 2016
https://dx.doi.org/10.7399/fh.2016.40.2.9268
Off-label prescription of drugs at hospital
Prescripción hospitalaria de medicamentos en condiciones fuera de ficha técnica
Vicente Arocas Casañ1, Jaime Mateo Carmona1, Olga García Molina1, M.a Ángeles Fernández de Palencia Espinosa1, M.a José Blázquez Álvarez1, M.a Amelia de la Rubia Nieto1 and Jesús del Río García2
1Pharmacy Unit, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia.
2Pharmacology Section, Universidad de Murcia.
ABSTRACT
Objectives: To develop a procedure for management of off-label medications, and to analyze the treatments, indications, and hospital units which will request them more frequently, as well as which variables will have an impact on the authorization decision, and its economic impact.
Methods: A procedure was designed where clinicians would complete request forms and the Hospital Unit would prepare reports assessing their efficacy, safety, convenience, and cost. The request forms for the past five years were analyzed.
Results: A total of 834 applications were received, and 88.1% of these were accepted. The authorization rates were higher for Paediatric Units (95.7% vs. 86.6%; p<0.05). The reasons for considering prescriptions as off-label were: different indication (73.2%), different combination (10.2%), different line of treatment (8.6%) and different age (8%). A 73.4% of requests were for antineoplastic drugs, and the most frequently prescribed were rituximab (120) and bevacizumab (103). The quality of evidence supporting the prescriptions was moderate-low, though no direct relationship with the likelihood of approval was demonstrated (p = 0.413). The cost of the approved medications was 8,567,537 €, and the theoretical savings for those drugs rejected was of 2,268,642 €. There was a statistically significant decrease in the authorization rate (p < 0.05, Student's t test) when spending increased.
Conclusions: The responsibility for assessing off-label prescriptions has fallen on the Pharmacy Unit. It has not been demonstrated that the quality of evidence represents a decisive variable for approval of treatment; on the other hand, age and cost have demonstrated a significant impact.
Key words: Off-Label Use; Medication prescriptions; Compassionate Use Trials.
RESUMEN
Objetivos: Desarrollar un proceso de gestión de medicamentos en condiciones fuera de ficha técnica y analizar los tratamientos, indicaciones y unidades clínicas que los solicitan, qué variables influyen en la decisión de autorización y su impacto económico.
Métodos: Se diseñó un procedimiento según el cual los clínicos cumplimentarían las solicitudes, el Servicio de Farmacia redactaría los informes valorando su eficacia, seguridad, conveniencia y coste, y la dirección médica tomaría la decisión de aceptar o no su uso. Se analizaron las solicitudes de los últimos cinco años.
Resultados: Se recibieron 834 solicitudes, autorizándose el 88,1%. Las tasas de autorización fueron mayores para los Servicios Pediátricos (95,7% frente a 86,6%; p < 0,05). Las razones por las que las prescripciones se consideraron fuera de ficha técnica fueron: diferente indicación (73,2%), combinación diferente (10,2%), línea diferente (8,6%) y edad diferente (8%). El 73,4% de las solicitudes fueron de antineoplásicos, siendo rituximab (120) y bevacizumab (103) los más prescritos. La calidad de la evidencia que avalaba las prescripciones fue moderada-baja, aunque sin demostrar relación directa con la probabilidad de aprobación (p = 0,413). El coste de los medicamentos aprobados fue de 8.567.537 € y el ahorro teórico de los denegados 2.268.642 €. El porcentaje de autorización disminuyó según aumentó el gasto de manera estadísticamente significativa (p < 0,05, test t de Student).
Conclusiones: La responsabilidad de evaluación de las prescripciones fuera de ficha técnica ha recaído en los Servicios de Farmacia. La calidad de la evidencia no ha demostrado ser una variable decisiva para la aprobación de los tratamientos. En cambio, la edad y el coste sí que han demostrado influir significativamente.
Palabras clave: Usos fuera de lo indicado; Prescripciones de medicamentos; Ensayos de uso compasivo.
Contribution to scientific literature
This is the most complete series on off-label prescription of drugs. Unlike other previous publications, all age groups and medical specialties have been included.
Our experience can encourage a higher number of Pharmacy Units to get involved in processes targeted to drive pharmacotherapy based on evidence, as suggested by Initiative 2020 from the Spanish Society of Hospital Pharmacy.
Introduction
Royal Decree 1015/2009, dated June, 19th, which regulates medication availability under special situations, establishes the access in Spain to off-label medications1.
Off-label use occurs in all medical specialties, but it is more frequent in those with a lower likelihood of patient inclusion in clinical trials (Paediatrics, Psychiatry, Obstetrics-Gynaecology)2,3. In an American study, 21% of the prescriptions for the 160 most widely used medications were off-label4. In Paediatrics, this has been observed in up to 80%5,6 of cases, in Oncology, in up to 50%7-11, and in Psychiatry, in up to 76%12,13. The majority presented little or no scientific evidence to support them14. There are few studies in Spain, and 22.3% off-label prescriptions have been observed in a recent publication15.
Until the approval of RD 1015/2009, the procedure to use off-label medications was clear and uniform; the Spanish Agency of Medicines and Medical Devices was responsible for authorizing the use of medications for Compassionate Use16-18.
Since the enforcement of RD 1015/2009, this responsibility has been transferred to the equivalent committees or bodies in each autonomous community, and the adaptation process initiated has led to the loss of this uniformity19,20.
In our community, until 2013 there was no regional committee that could take responsibility for this new situation; therefore, each hospital had to design their own procedure of action21.
The main objective of this Project was to develop a procedure for management of medications prescribed off-label in the hospital setting, in order to adapt to RD 1015/2009 and, at the same time, to meet the Objective 2.3 from Initiative 2020 of the Spanish Society of Hospital Pharmacy (SEFH): The Pharmacy Unit will be actively involved in programs with the objective of treating patients with pharmacotherapy based on evidence22.
As secondary objectives, first we intended to analyze which the most common treatments and indications were, and the clinical units which requested them more frequently; secondly, to research which variables of patients or treatments had an impact on the decision of authorization for a drug; and finally, which was the economic impact of the authorized drugs, and the theoretical spending represented by the use of the rejected drugs.
Methods
In order to adapt to RD 1015/2009, a Standard Operating Procedure (SOP) was designed, based on the one designed by the GENESIS Group (Group for Innovation, Assessment, Standardisation and Research in the Selection of Drugs) from the SEFH23. This SOP was approved by the Pharmacy & Therapeutics Committee (PTC) and Medical Management of the hospital, which accepted the responsibility for authorizing treatments, with technical support by the Area of Medication and Selection of Drugs (AMSD) from the Pharmacy Unit.
According to this SOP, the clinician should complete a request form with identity data, previous treatments, alternative treatments, and reasons justifying the requested treatment, attaching any bibliographic references available.
The AMSD would have seven days to prepare an evaluation report, with a study of the efficacy, safety, convenience and cost of the treatment requested and its alternative options, according to the specific situation of the patient, with a proposal for approval or rejection by the Hospital Management.
This analysis includes the individualized requests received during the past five years, from October, 1st, 2009 until September, 30th, 2014, both for hospitalized patients and outpatients.
The study did not include those treatments subject to off-label protocols from the time of their approval by the hospital PTC, as well as individualized off-label treatments from the time of receiving the indication by Regulatory Agencies. The request forms received were reviewed for this analysis, as well as the individualized evaluation reports prepared by the AMSD of the Pharmacy Unit, and the clinical records whenever it was necessary.
The authorization rate of treatments was considered as the primary variable, and was analyzed based on patients' demographic data (age and gender), the requesting clinical department, the drug or combination of drugs, the indication for which the treatment was requested, the reason to consider treatment as off-label, the evidence regarding efficacy and safety available at the time of writing the report, and the cost of treatment.
The causes for treatments to be considered off-label were divided into four groups:
1. Indication not approved at the Product Specifications (PS). When the specific condition requested did not coincide with the one described in the PS.
2. Indication not approved for the patient's age. When the patient did not fall into the specific age group targeted by the treatment, according to the indication described in the PS, or when this age group had been explicitly excluded.
3. Indication for a line of treatment different to the one approved in the PS. When a specific line of treatment was stated in the PS, which did not coincide with the line requested.
4. Prescription within a combination of drugs different to the one approved in the PS. When a specific combination of drugs was stated in the PS, which did not coincide with the one requested.
Evidence was classified according to the GRADE system (Grading of Recommendations, Assessment, Development and Evaluation), into High, Moderate, Low, and Very Low Evidence24.
• High Evidence: meta-analysis, randomized clinical trial, systematic review of randomized clinical trials.
• Moderate Evidence: controlled clinical trials well designed but not randomized, randomized clinical trials in another line of treatment which could be extrapolated, randomized clinical trials in another population which could be extrapolated, studies of cohorts or cases and high quality controls and multicenter.
• Low Evidence: studies of cohorts or cases and controls, multiple series compared over time.
• Very Low Evidence: series of cases, experts' opinion.
In order to calculate the cost, the duration of treatment was considered according to the studies available and the cost of acquisition by the hospital. For durations over 12 months, the estimation was exclusively for the cost of the first year. The real price of acquisition by the hospital (selling price - discounts + VAT) was used for calculation. Other direct medical costs associated were not quantified, such as administrative costs, and hospital stays; indirect costs were not quantified either, though all of these were taken into account in a qualitative manner for decision making.
The SPSS Program for Windows, version 19.0, was used for data analysis. The statistical relationship between scientific evidence and the decision by the Hospital Medical Management, and the relationship between the authorization rate between clinical units for adults and paediatrics, were calculated through Χ's Test2. The relationship between cost and Hospital Medical Management decisions was calculated through Student's t test. The statistically significant value was p<0.05, and the Bonferroni Correction was used to adjust the alpha level, preventing false positive results. For this aim, p<0.05/number of comparisons was taken as statistically significant.
Results
The SOP designed was widely followed at hospital, and 834 individualized requests for off-label treatments were received during the period of the study. Out of these, 88.1% were authorized. 51.3% of them were for male patients. The median age of patients was 50 years (Table 1).
Regarding the reason that led to the treatment being considered off-label, the majority of requests (73.2%) were due to indication not approved in the PS, and 87.5% of these were authorized. When the request was conducted because the age of the patient was different to the one in the Product Specifications (8% of requests), 100% of requests were authorized. The lowest rate of authorizations was for those requests for treatment in a different line to the one authorized (8.6% of requests, and 75% of authorizations) (Table 2).
Regarding the level of evidence available at the time of the request, Table 3 sums up its distribution. The levels of evidence were compared regarding the decisions for authorization or rejection, and no significant differences were observed (p=0.413, chi-squared test2).
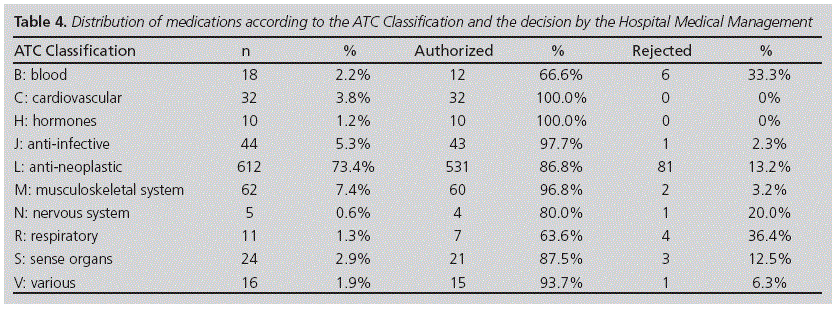
The distribution of medications requested was conducted according to the ATC classification (Table 4). It was observed that the most requested group of medications were antineoplastic, with 73.4% (612). The drugs with the highest number of requests were: rituximab (120), bevacizumab (103), and bendamustine (65) (Figure 1).
The most repeated indications were glioma (72), B cell lymphoma (46) and epithelial ovarian cancer (33). The most frequent indications appear on Table 5, with their respective decision by the Medical Management.
83% of the requests came from clinical units for adults, and 17% from Paediatrics. The distribution of requests in terms of clinical units appears on Tables 6 and 7.
The authorization rate for requests in the paediatric units (95.7%) was higher than in the units for adults (86.6%) (p<0.05, chi-squared test2).
The theoretical cost represented by the approved medications was calculated, and it reached a total amount of 8,567,537 €. The total expense in Pharmacy during the period of the study (October, 2009 to September, 2014) added up to 250,702,423 €. Therefore, the expense in off-label drugs represented 3.42% of the total expense in Pharmacy. The median cost per patient was 8,544 € [1,970, 16,101]. It was observed that treatments with costs between 0 and 1,000 € were authorized in 96.8% (123) of cases, while those with costs between 50,000 and 100,000 € were only authorized in 50% of cases (12) (Table 8).
The authorization rate decreased as the spending range increased, in a statistically significant way (p<0.05, Student's t).
The rejected treatments, in case they had been approved, would have caused an expense of 2,268,642 €, with a median cost per patient of 14,010 € [4,684, 35,935]. This would have represented the 0.90% of the amount spent in medications during that period.
Discussion
We believe this is the largest and most complete study conducted since the change of legislation approved in September, 2009. It covers a period of five years, and includes information from over 800 requests for treatments prescribed off-label.
In our country, there are few studies about off-label use of medications15,25. There are descriptive studies about the authorization rates for off-label medications in hospital, but with a low number of requests studied. Our authorization rates are higher than those described by Pérez-Moreno in the Hospital Virgen del Rocío (88.1% vs. 60.8%), though in that case the duration of the study was under 2 years, and only included 51 requests. Regarding the clinical units which present more requests, the results are similar: oncohaematological units in both studies20.
Though various studies have been published in other countries, these don't consider the Spanish legislation4. In other occasions, studies have collected partial aspects of therapy, such as oncological treatments26, treatments in the paediatric population27,28 or only some groups of drugs29,30. Our study includes all medical specialties and population groups seen in a third-level university hospital.
Regardless of the high number of requests processed during this period, this procedure could only be applied to those medications which present a higher level of control by the Hospital Pharmacy: medications with restricted use, cytostatics with high economic impact, medications dispensed to outpatients, and drugs not included in the Hospital Formulary. This is a major limitation, because for example, there is published evidence that prescription in Paediatrics is mostly conducted off-label6,31. We find another example in the prescription of chemotherapy treatments, because according to the American Society of Cancer, almost half of the prescriptions for cytostatic medications are not written according to their PS10.
Against what could be expected, our study does not demonstrate a direct relationship between the quality of the evidence published and the likelihood of approval for a medication. We can find a reasonable explanation for this in the fact that, even though a treatment might be supported by major studies, there are often other treatments with the same evidence of efficacy and safety at a lower cost. In other cases, on the contrary, even though there was little evidence, the lack of other therapeutic alternatives led to the approval of treatments supported only by a reduced series of cases, or even by isolated cases. On the other hand, for some indications, such as hyperactive bladder, all treatments requested were authorized, because there was solid evidence available. In other cases, the need to initiate treatment as soon as possible, due to the severity of the clinical condition, led to the urgent approval of all cases (optic neuromyelitis, acute humoral rejection).
On the other hand, it has been demonstrated that cost has a direct relationship with likelihood of approval. This relationship appears logical, because at a higher cost, there is a higher likelihood that the incremental cost-efficacy will be above the commonly accepted thresholds.
Another variable which has demonstrated high impact upon the decision by the Medical Management is the fact that the cause for the treatment to be considered off-label was the age of the patient. Typically, clinical trials will be conducted mostly with adult patients. Therefore, some treatments with a high experience of use in adults have no approved indication for the paediatric population. In our series, all treatments of this type were approved, regardless of the quality of evidence available in the paediatric population. On the other hand, this massive approval of treatments did not represent a major economic impact, because even though the number of requests added up to 8.0% of the total, the cost was limited to 254,870 €, a 2.97% of the theoretical amount for the authorized treatments.
On the contrary, when the cause for the drug to be considered off-label was its use in a different line than the one approved, the rate of rejections was significantly higher than for the rest of scenarios. The high number of treatments for breast cancer rejected stands out; in the majority of cases, this is due to the availability of other treatments which are more cost-effective and have their indication approved in the product specifications.
Another problem we found was the classification of the available evidence. The fact of not having the approved indication presents a direct relationship with the lack of well-designed studies supporting the efficacy and safety of a treatment. The lack of large randomized studies was a regular constant. Finally we decided to use the GRADE23 system, because we considered that it was the most adequate to fit our needs.
Besides, evidence was changeable, because during the period of five years covered by our analysis, new evidence kept constantly appearing. Some treatments that had been initially processed as off-label finally received the approval of their indication by the EMA. This was the case, for example, with everolimus for breast cancer, with botulinum toxin for urinary incontinence, or with bevacizumab for ovarian cancer.
There have also been other variables difficult to control, which had an impact and were not recorded in this study, such as the availability of therapeutic alternative options for the treatment requested, clinical situation of the patient, pressure by relatives, insistence by the prescribing physician, urgency of treatments, etc.
Though it was intended that the procedure of preparation of Therapeutic Use Reports was as homogeneous and reproducible as possible, the personal variability of the authors of each of these reports must be taken into account. All resident pharmacist on rotation in the AMSD from October, 2009 to September, 2014 have participated in these reporting. Even though all reports were reviewed by the pharmacist responsible for the area before being submitted to the Medical Management, the fact that they were written by many different authors might have had an impact on their homogeneity.
Another potential limitation in our study could be the lack of record of the clinical outcomes achieved with the treatments administered, which would help to assess their efficiency.
In our Hospital Pharmacy Unit, the change of legislation in 2009 has increased noticeably the AMSD activity. The saving in 2.2 million € which have not been spent on treatments with little efficacy or with more adequate therapeutic alternatives, demonstrates, once more, the high additional value of the Hospital Pharmacy Unit by achieving an individualized therapy with high quality, safe and effective, thus fulfilling the mission of Hospital Pharmacy Units.
Bibliography
1. Real Decreto 1015/2009, de 19 de junio, por el que se regula la disponibilidad de medicamentos en situaciones especiales. Boletín Oficial del Estado, no 174, (20 de julio de 2009). [ Links ]
2. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-90. [ Links ]
3. Danes Carreras I, Vallano Ferraz A, de la Cruz Sugranes G, Juarez Giménez JC, Arnau de Bolos JM. Utilización de medicamentos y condiciones de uso recomendadas en pediatría. An Esp Pediatr. 2002;57(5):414-9. [ Links ]
4. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-6. [ Links ]
5. Shah SS, Hall M, Goodman DM, Feuer P, Sharma V, Fargason C Jr, et al. Off-label drug use in hospitalized children. Arch Pediatr Adolesc Med. 2007;161(3):282-90. [ Links ]
6. Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164 (9):552-8. [ Links ]
7. Leveque D. Off-label use of anticancer drugs. Lancet Oncol. 2008;9(11):1102-7. [ Links ]
8. Roila F, Ballatori E, Labianca R, De Braud F, Borgonovo K, Martelli O, et al. Off-label prescription of antineoplastic drugs: an Italian prospective, observational, multicenter survey. Tumori. 2009;95(6):647-51. [ Links ]
9. Pearson SA, Ringland CL, Ward RL. Trastuzumab and metastatic breast cancer: trastuzumab use in Australia-monitoring the effect of an expensive medicine access program. J Clin Oncol. 2007;25(24):3688-93. [ Links ]
10. The American Cancer Society. Off-label drug use. (Página Web). Estados Unidos. (Consultado 15/05/2015). Disponible en: http://www.cancer.org/Treatment/TreatmentsandSideEffects/Treatment-Types /Chemotherapy/off-label-drug-use [ Links ]
11. Kalis JA, Pence SJ, Mancini RS, Zuckerman DS, Ineck JR. Prevalence of off-label use of oral oncolytics at a community cancer center. J Oncol Pract. 2015;11(2):e139-43. [ Links ]
12. Martínez-Lazcano MT, Esplá-González S, Herraiz-Robles P, Hernández-Pérez P, Chillerón-Cuenca R, Pol-Yanguas E. Use of valproic acid in long stay units of psychiatry. Farm Hosp. 2015;39(2):92-101. [ Links ]
13. Kharadi D, Patel K, Rana D, Patel V. Off-label drug use in Psychiatry Outpatient Department: A prospective study at a Tertiary Care Teaching Hospital. J Basic Clin Pharm. 2015; 6(2):45-9. [ Links ]
14. Mellor JD, Van Koeverden P, Yip SW, Thakerar A, Kirsa SW, Michael M. Access to anticancer drugs: many evidence-based treatments are off-label and unfunded by the Pharmaceutical Benefits Scheme. Intern Med J. 2012;42(11):1224-9. [ Links ]
15. García-Sabina A, Rabunal Rey R, Martínez-Pacheco R. Revisión sobre el uso de medicamentos en condiciones no incluidas en su ficha técnica. Farm Hosp. 2011;35(5):264-77. [ Links ]
16. Monedero MA. Uso compasivo. En: Suñé JM, Bel E. Formación Continuada para Farmacéuticos de Hospital. 1a Ed. Barcelona: Ferrer Grupo; 2001. p. 109-16. [ Links ]
17. Real Decreto 223/2004, de 6 de febrero, por el que se regulan los ensayos clínicos con medicamentos. Boletín Oficial del Estado, no 33 (7 de febrero de 2004). [ Links ]
18. Ley 29/2006, de 26 de julio, de Garantías y Uso Racional de los Medicamentos y Productos Sanitarios. Boletín Oficial del Estado, no 178 (27 de julio de 2006). [ Links ]
19. Delgado O, Puigventós F, Clopés A. Position of the hospital pharmacist regarding the use of medication in non-authorised conditions. Farm Hosp. 2009;33(5):237-9. [ Links ]
20. Pérez-Moreno MA, Villalba-Moreno AM, Santos-Ramos B, Marín-Gil R, Varela-Aguilar JM, Torello-Iserte J, et al. Off-label approval of drug use in a tertiary hospital. Rev Calid Asist. 2013;28(1):12-8. [ Links ]
21. Orden de 26 de julio 2012 de la Consejería de Sanidad y Política Social por la que se crean y se establece la composición, organización y funcionamiento del Comité Regional de Evaluación de Tecnologías Sanitarias y de la Comisión Regional de Farmacia. Boletín Oficial de la Región de Murcia, no 182 (7 de agosto de 2012). [ Links ]
22. Grupo 2020. Objetivos 2020. (Monografía en internet). Madrid. Sociedad Española de Farmacia Hospitalaria; (citado 15/04/2015). Disponible en: http://gruposdetrabajo.sefh.es/2020/index.php?option=com_wrapper&view=wrapper&Itemid=2 [ Links ]
23. Grupo GENESIS. Propuesta GENESIS de PNT para la utilización en el hospital de medicamentos fuera de indicación, fuera de ficha técnica u off label. (Monografía en Internet). Madrid: Sociedad Española de Farmacia Hospitalaria; 2009 (Consultado 15/04/2015). Disponible en: http://gruposdetrabajo.sefh.es/genesis/genesis/Enlaces/PNT_FFT_GENESIS_Borrador_07 _11_2009.pdf [ Links ]
24. GRADE working group. GRADE. (Página Web). (Consultada 10/04/2015). Disponible en: http://www.gradeworkinggroup.org/ [ Links ]
25. Lozano Ortiz R. Drug use in off-label dosage regimes. Farm Hosp. 2015;39(2):122-6. [ Links ]
26. Conti RM, Bernstein AC, Villaflor VM, Schilsky RL, Rosenthal MB, Bach PB. Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. J Clin Oncol. 2013;31(9):1134-9. [ Links ]
27. Morales-Carpi C, Estan L, Rubio E, Lurbe E, Morales-Olivas FJ. Drug utilization and off-label drug use among Spanish emergency room paediatric patients. Eur J Clin Pharmacol. 2010;66(3):315-20. [ Links ]
28. Ruiz-Antorán B, Pineiro R, Avendano C, Roman E, Cilleruelo ML, Gutierrez-Junquera C, et al. Drug utilization and off-label drug use in Spanish pediatric gastroenterology outpatients. J Pediatr Gastroenterol Nutr. 2013;56(2):173-7. [ Links ]
29. Porta A, Espósito S, Menson E, Spyridis N, Tsolia M, Sharland M, et al. Off-label antibiotic use in children in three European countries. Eur J Clin Pharmacol. 2010;66(9):919-27. [ Links ]
30. Fukada C, Kohler JC, Boon H, Austin Z, Krahn M. Prescribing gabapentin off label: Perspectives from psychiatry, pain and neurology specialists. Can Pharm J (Ott). 2012;145(6):280-4. [ Links ]
31. Bazzano AT, Mangione-Smith R, Schonlau M, Suttorp MJ, Brook RH. Off-label prescribing to children in the United States outpatient setting. Acad Pediatr. 2009;9(2):81-8. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: vicentearocas@hotmail.com
(Vicente Arocas Casañ).
Recibido el 14 de mayo de 2015;
aceptado el 9 de diciembre de 2015.











 texto en
texto en