Contribution to scientific literature
SiMON-VC is the first information system developed for the pharmacotherapeutical monitoring of patients with Chronic Hepatitis C which enters automatically those data included in the electronic clinical record.
It allows a more efficient and safe pharmacotherapeutical follow-up, as well as an on-going improvement in healthcare quality; it promotes coresponsibility in healthcare outcomes, and it facilitates research in health outcomes.
Introduction
The use of new information technologies in order to improve healthcare of the citizens is one of the strategic lines of the Quality Plan for the National Health System1, which has been the basis for the development of the different healthcare strategies by the Spanish regional healthcare systems. In Galicia, the Strategy by the Servicio Galego de Saúde 20142 incorporates this strategic line, which has been the basis, among other actions, for the development of a single electronic clinical record for the entire regional Primary Care and hospital setting, or for the use of the computerized physician prescription in 100% of the population.
In our hospital, and as part of this strategy, the Information Systems Unit has developed an information system with a specific technological setting (Table 1), named with the acronym SiMON, for “Sistema inteligente de MONitorización de pacientes” (Intelligent System for Patient Monitoring); its objective is to increase the efficacy in patient care and help physicians and pharmacists to make decisions, through the automation of the information available about the product at each time point within the hospital setting. The Pharmacy Unit has joined this system (Table 2), through the implementation of three protocols already in production (follow-up of patients with Chronic Hepatitis C on antiviral treatment, prescription-validation-preparation of parenteral nutrition formulations, and traceability of biologics through radiofrequency), and one under development (pharmacokinetics, pharmacogenetics).
Table 2 SiMON protocols in production and under development (those implemented from the Pharmacy Unit are in cursive letter)

A core activity by Hospital Pharmacists in Spain consists in Pharmaceutical Care (PhC) for outpatients; in our hospital, this is based on national and international standards, including the Strategy by the Servicio Galego de Saúde 20142, the Quality Plan and Quality Policy of the Hospital, the Quality Handbook by the Pharmacy Unit, and the SEFH 2020 Initiative “Hacia el futuro con seguridad” (Towards the Future with Safety)3. Based on these fundaments, the Hospital Pharmacy Unit has considered various strategic axes, including Care Quality and Safety and Information Systems and Communication Technologies.
In the setting of PhC for outpatients, these axes have led to opening Monographic Units for Outpatient Pharmaceutical Care, as a central action line; 16 of these units have been established nowadays, managed managed by a Specialist Pharmacist subspecialized in this monographic area. Since the enforcement of the National Strategic Plan for Addressing Hepatitis C in April, 20154, a Monographic Pharmaceutical Care Unit for Patients with Chronic Hepatitis C was created, based on a preexistent Monographic Unit for Viral Conditions (HIV, HCV, HBV). One of the objectives of this unit was to develop an Information System to promote:
The quality and efficiency of PhC, through an automatic system for patient information associated with the Outpatient Pharmacy Unit.
Patient safety during antiviral treatment.
Research on health outcomes.
And under these premises, an Intelligent System for Monitoring Clinical Virology (SiMON-VC) was developed, in collaboration with the Information Systems Unit and the Internal Medicine Unit responsible for the medical follow-up of patients with this condition.
The objective of this article is to describe the requirements, structure and features of SiMON-VC.
Method
The design of this information system for therapeutical monitoring and research of HCV patients on antiviral treatment was developed by the Pharmacists responsible for Pharmaceutical Care in the Monographic Outpatient Unit and with the computer technicians in the Information Systems Unit, during bi-weekly meetings over the period of one year.
The final conclusion was that the design of SiMON-VC should allow to:
Record patients with Chronic Hepatitis C on treatment, with automatic incorporation of basal and epidemiological data of the patient from their electronic clinical record.
To record PhC followups in the Monographic Unit, automatically incorporating blood, biochemical and microbiological tests from the Lab databases.
To generate events and automatic pharmaceutical alerts regarding the efficacy and safety of pharmacological treatment.
To estimate patient adherence to antiviral treatment.
To document Pharmacy visits in the electronic clinical record, by exporting the records of Pharmaceutical Care.
To generate an Activity Control Panel and a Quality Control Panel, with automatic generation of indexes and alerts about the standards determined.
Statistical treatment of Big Data through Business Intelligence (BI) tools.
Statistical treatment of Big Data through Business Intelligence (BI) tools.
Maximum versatility in the use of data.
Based on these requirements, SiMON-VC was designed with the structure and performance features detailed below.
Results
SiMON-VC structure
SiMON-VC features a Main Menu shown in Figure 1, which can be accessed by the individualized passwords for each Pharmacist; this menu has been structured into two sections containing the following tabs with different functions:
-
Upper section (grey background)
- Insert record: It allows to enter each antiviral treatment course for a patient.
- Agenda: Shows a list of patients with previous appointment for the Pharmaceutical Care Unit.
- Search patient, Search by ID, List: Tabs to locate registered patients.
- Control Panel: Incorporates activity and quality indexes.
- Insert monitoring: Generates a record of the objective and subjective data generated in each one of the patient visits.
- Sign: Records electronically every follow-up visit in the electronic clinical record.
- Import: Allows access to all predetermined data in the clinical patient record.
- Reports: Generates reports with data selected by the Pharmacist.
-
Lower section (white background, left side): After each patient record, it enters in the form of a tree the following options:
Lower section (White background, right side): It shows the contents of each option in the tree. It is described below.
a) Main record
Each patient is assigned a Main Record for each antiviral treatment course. As can be observed in Figure 1, this is structured into three tabs, where the majority of data from the electronic clinical record are automatically entered:
-
Record data:
-
Basics:
-
Epidemiology:
- Year of diagnosis and infection transmission pathway.
- Co-infection with HAV, HBV, HIV (yes/no). Syphilis (yes/no).
- HCV genotype and IL28B polymorphism.
- Response to previous treatments (naïve, relapsing, partial response, no response, unknown previous response).
- Antiviral treatment: Initial and final dates.
- Initial and final fibrosis.
- Sustained Virological Response (SVR: yes/no).
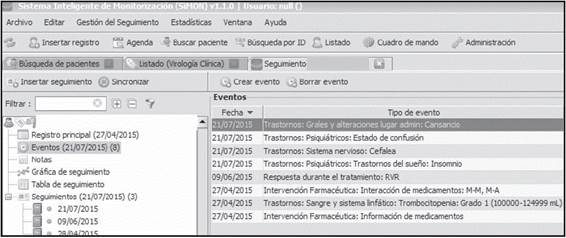
b) Events
One of the most important functions in SiMON-VC is based upon the Events associated with treatment efficacy and safety or pharmaceutical actions. Events are predetermined through algorithms in the computer structure of SiMON-VC, and after incorporating the defining data automatically from the electronic clinical record, they appear as an alert in the computer screen. They can be entered and subsequently processed, either through an Indicator Control Panel, or statistically for research studies.
b.1. Treatment Efficacy Events
Regarding treatment efficacy, SiMON-VC includes different types of response in a coded manner, which will appear as an event/alert when data from the Microbiology Lab are automatically entered in a follow-up visit. Events are defined as the efficacy variables from the antiviral treatment against HCV: RVR-Rapid Virological Response (undetectable HCV RNA at week 4 of treatment), EVR-Early Virological Response (undetectable HCV RNA at week 12 of treatment), FVR-Final Virological Response (undetectable HCV RNA at the end of the treatment), and SVR-Sustained Virological Response (undetectable HCV RNA at week 12 after completing the treatment). For example, if a patient presents negative HCV virological load at week 4 of antiviral treatment, SiMON-VC will automatically generate the event “Rapid Virological Response-RVR”, it will be registered as such, and it can be used for patient care or research purposes, because it is a “coded” computer record.
At the same time, alerts have been included for Discontinuation Rules of the antiviral treatment, during the use of dual therapy based on Pegylated Interferon + Ribavirin, and triple therapy based on first-generation Protease Inhibitors.
b.2. Treatment safety events. Alerts
Events associated with treatment safety are entered manually during the clinical interview with the patient by the Pharmacist in the Outpatient Unit, selecting those adverse events reported by the patient from a predefined database that includes all adverse events described for the antiviral treatment.
Safety events are also automatically generated, which appear as SiMON-VC alerts, with the objective of being support systems for decision making. For example, events associated with potential contraindications of the antiviral treatment according to the basal data of the patient, lab data from the patient, or events associated with incorrect dosing. Based on event generation the system will suggest a recommendation or pharmaceutical action according to the alert issued.
Safety alerts for haematological adverse events have also been predefined according to their severity, and these are automatically generated and entered based on the blood parameters of the patient. Thus, for example, if a patient presents a haemoglobin level of 8 g/dL in a specific follow-up visit, the system will generate a “Grade III Anaemia” alert, and record it as an event.
Alerts for clinically significant interactions have also been predefined for each one of the medications used.
b.3. Pharmacist Action Events
Another type of coded events are those associated with Pharmacist actions derived from the clinical interview between Pharmacist and patient, and from the safety alerts generated in the visit by the patient. In total, 12 types of Pharmacy Interventions have been coded.
As an example, Figure 2 shows the events generated in that specific date for a patient throughout their pharmacotherapeutical follow-up, and associated with treatment efficacy (RVR: Rapid Virological Response), safety (Grade 1 Thrombocytopenia, fatigue, confusion, headache, insomnia) or active pharmaceutical actions such as drug-drug interaction, drug-food interaction, or information on medications.
c) Notes
This is an uncoded free-text field (and therefore, it cannot generates a computer network exploitation), where the Pharmacist enters various aspects associated with patient follow-up (for example: “reinforce information in the next visit”, “analyze digoxin pharmacokinetics by pharmacological interaction”, etc.).
d) Monitoring Graphs
This SiMON-VC option allows to design temporary graphs that will show the evolution of patient parameters and treatments throughout all the monitoring conducted:
Hepatic fibrosis grade of the patient.
Treatment adherence.
Blood count and biochemical tests.
Child-Pugh Score.
HCV viral load.
e) Monitoring Table
This is the table option by monitoring date, which allows the design of the Monitoring Graphs previously described. Figure 3 shows the Monitoring Graph for platelet count recovery of a patient throughout their treatment.
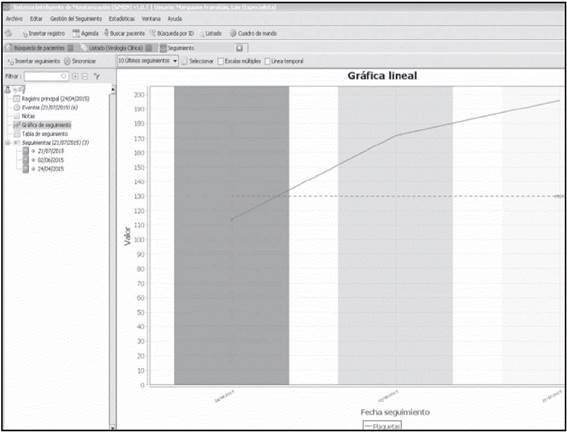
Figure 3 Monitoring graph for the platelet count evolution of a patient throughout their pharmacotherapeutical follow-up.
f) Follow-up Visits
The essential option in SiMON-VC is Follow-up Visits, where the greatest part of the information for the system is generated for each patient during their pharmacological treatment. The number of Follow-up Visits for each patient is equal to their number of visits to the Pharmacy Unit, including the first one on the day of treatment initiation, and the last follow-up visit (and closure of the patient record), at 12-24 weeks after the day of pharmacological treatment completion (with the objective of recording the 12-24 Sustained Virological Response).
Each Follow-up Visit is structured into three tabs, where the greatest part of the electronic clinical record data are entered for the date of that visit; there is stratification by colours of the values based on the normality range, information about the variation in the value com pared with the previous follow-up visit, and an alert is generated if a predefined rule is met:
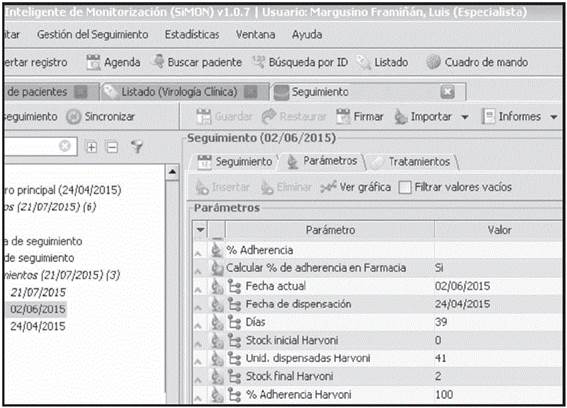
The follow-up for the Treatment Adherence parameter presents a particular interest in the monographic PhC visit; this is automatically generated in each patient visit, by manually entering the number of remaining dosing units on the day of the visit. An algorithm has been designed to enter the date of the follow-up visit (current and previous), medication units dispensed, and medication stock (current and previous), and the result obtained is the percentage of Treatment Adherence by medication (Figure 4).
SiMON-VC features
The main feature of SiMON-VC is being a Pharmaceutical Care supporting system for patients with Chronic Hepatitis C, as shown in the previous chapter describing its structure. Besides, SiMON-VC presents the following features that are developed in a completely versatile setting:
Report Generation.
Pharmaceutical Care Documentation.
Control Panel for Activity, Quality and Safety.
Research.
a) Report Generation
SiMON-VC issues reports based on data from the main record or the follow-up visits. Its versatility and autonomy allows selecting the type of report and presenting them as tables or graphs, with transversal or longitudinal monitoring.
b) Pharmaceutical Care Documentation
PhC documentation provided by the Pharmacist for each visit at the Outpatient Pharmacy Unit can be entered into the electronic clinical record by exporting to the clinical course of the patient all monitoring and reports previously recorded and signed by the Pharmacist in charge with their personal password, in order to enter it as a document on its export date. This function has a particular clinical interest in terms of the monitoring, secondary to detecting clinically significant interactions, and monitoring antiviral treatment adherence.
c) Control Panel for Activity, Quality and Safety
An essential aspect of the program is its potential to design, in a completely automatic and versatile manner, a control panel for Activity, Quality or Safety Indicators, to obtain indexes and generate alerts when previously determined standards are not met. This turns SiMON-VC into a Support System for Decision Making. As can be observed in Figure 1, the “Control Panel” option is part of the Main Menu in SiMON-VC.
This option also features a self-control system for quality of compliance with the essential basal data, which allows to identify directly those patients for whom these data have not been adequately recorded.
For example, Figure 5 shows the Control Panel in SiMON-VC for the first 2016 trimester, where two alerts for lack of protocol compliance can be seen (ninth line) relative to the proportion of patients with Grade 2 liver fibrosis, and the proportion of patients on a specific antiviral treatment (last line).
d) Research
A strength in this information system is the potential use with research purposes of a high amount of recorded and coded data that are entered or automatically generated in SiMON-VC, at the evaluation level of health outcomes derived of the use of medications and/or the PhC provided, through Big-Data and BI tools. Data can be exported to a file compatible with most statistical tools (Excel®, SPSS®…).
The program automatically generates graphs representing the analysis outcomes of data predetermined by the user, both for the overall population registered and for specific patient profiles. Besides, it can create or modify through BI all types of population analysis, for patient record data as well as for any of the follow-up visits conducted until the end of Pharmaceutical Care in the Outpatient Unit, as shown in the next chapter.
SiMON-VC was implemented on January, 1st, 2012, after a previous validation period, and since then it is the information system used in the PhC Monographic Outpatient Unit for the record and follow-up of Chronic C Hepatitis patients on antiviral treatment. Table 3 shows the basal record data of those patients who initiated an antiviral treatment course between April, 1st, 2015 and April, 1st, 2016 (1 natural year since the implementation of the National Strategic Plan for Addressing Chronic Hepatitis C by the Spanish Government). During this period, 665 patients have initiated antiviral treatment against HCV, and 2,493 follow-up visits have been conducted.
Table 3 Basal characteristics of patients included in SiMON-VC during the first year after the approval of the National Strategic Plan for Addressing Chronic Hepatitis C (enforced on April, 1st, 2015)

During this follow-up, automatic and manual events have been generated and entered for each patient, associated with antiviral treatment efficacy and safety, or pharmaceutical interventions. Table 4 includes the main events during the analyzed period.
Discussion
Pharmaceutical Care for outpatients provided by Pharmacy Units in Spanish hospitals is an activity that had its main driver in 1991, with the change of the prescription and dispensing setting for different medicinal products in the National Health System(5). Since then, this activity has gradually increased year after year, and different national and international positions have been stated, that have guided Hospital Pharmacists regarding the objectives and procedures to follow in its development (6-14), putting forward a series of strategies to encourage patient-centered Pharmaceutical Care, collaboration in multidisciplinary teams, documentation of the care provided, and joint responsibility in health outcomes.
More recently, in the past two decades, different initiatives have been driven by the professional associations of reference in the Spanish Hospital Pharmacy setting; these have encouraged new strategic lines to be implemented in general Hospital Pharmacy practice, and particularly in the outpatient practice, such as the development of new technologies and integrated information systems, patient care quality and safety, and research(15-20). Aligned with these strategic lines, Spanish Hospital Pharmacy Units have developed numerous actions focused on the implementation of Assisted prescription programs for Computerized Physician Order Entry, both for hospitalized patients and outpatients, following the initiatives by the Spanish Society of Hospital Pharmacy(21); they have driven the certification by authorized companies of the quality developed, and they have encouraged research, particularly in the field of health outcomes by new pharmacological treatments.
However, Pharmacotherapeutical Monitoring is a core procedure in the Hospital Pharmaceutical Care area, where no tools have been developed to meet these strategic lines previously mentioned (information systems, quality-safety and research). In this sense, SiMON-VC is the first integrated information system developed in Spain that facilitates the pharmacotherapeutical monitoring of patients under the responsibility of the Hospital Pharmacist, specifically outpatients infected with Hepatitis C virus who are on antiviral treatment.
As has been shown in the previous sections about the structure and features of SiMON-VC, this information system meets all requirements initially proposed for its development, by maximizing the quality of the Pharmaceutical Care provided in the Outpatient Pharmacy Unit, and increasing antiviral treatment safety.
Firstly, SiMON-VC is a very versatile information system constantly updated, because it is developed through the on-going collaboration between pharmacists and physicians responsible for HCV patient care and the Hospital Information System Units. This allows to avoid unnecessary latency periods at the time of updating medications, clinical variables, or new alerts issued by Spanish or European regulating agencies. And at the same time, it strengthens the multidisciplinary collaboration of healthcare professionals managing the patient, not only through its technological development, but also through its joint use in patient visits to the physician and the pharmacy.
This system is integrated with the rest of information systems in the hospital, which allows the automatic entry of all parametric data in the rest of the hospital information systems (admission, biochemistry, blood count, coagulation, microbiology, anatomopathology, etc.), thus reducing as much as possible the time required and the potential errors derived from the manual entry of variables. All information systems used by all hospital units can be integrated with SiMON-VC.
Through the Record, Basics and Epidemiology Data from the Main Record, SiMON-VC generates a database including all HCV patients seen in the Pharmacy Unit, with those records required for a subsequent joint or stratified use of patient data for research purposes.
Some essential aspects in the pharmacotherapeutical monitoring of outpatients are: assessment of treatment efficacy and safety, adherence to the treatment dispensed in the Pharmaceutical Care Unit, or drug-drug or drug-food interactions. SiMON-VC incorporates all these aspects, which can be automatically included from the clinical record or manually entered during the Pharmacist-patient clinical interview; the required variables include HCV viral load before and during treatment and after its completion, lab values (biochemical, blood tests…), records of dispensing and return of medications or concomitant medications. And also from the perspective of Pharmaceutical Care, SiMON-VC allows to enter all Pharmacist actions provided to the patient.
A key aspect of the information system from the perspective of patient care safety is the previous definition and automatic generation of safety alerts during the Pharmaceutical Care visit, both regarding indication-contraindication, clinically significant interactions, or dosing regimen of the pharmacological treatment, and therapy outcomes in terms of safety (for example, development of severe haematological effects) and efficacy (for example, lack of response or virological relapse). The alerts generated allow the Pharmacist to take proactive actions regarding pharmacological treatment (dose adjustment, recommendation for pharmacokinetic monitoring, or close blood pressure monitoring in case of interactions, for example) in collaboration with the hospital physician responsible for the patient as well as with their Primary Care physician or pharmacist.
On the other hand, the potential to design and generate predefined reports with the relevant information about pharmacotherapeutical monitoring (for example, graphs of adherence to antiviral treatment, development of adverse effects, or specific recommendations) and their export through digital signature to the electronic clinical record, will not only allow to document the Pharmaceutical Care provided, but also to share this information with the rest of the healthcare team managing these patients.
Moreover, SiMON-VC is a support system for decision making, which allows to define an Indicator Control Panel, with alerts when those standards determined are not met in terms of demographical data, antiviral treatment, etc. This function is widely used as a tool for the economic management of a very effective but undoubtedly costly treatment, because it allows to know not only the number of patients treated during the period of time selected, but also their stratification according to type of patient (HIV coinfection, transplant, initial fibrosis, previous treatment…) or type of antiviral treatment prescribed.
Finally, as described in the Features Section, SiMON-VC is an extremely potent tool for using codified data for research purposes, through the development of the Big-Data and BI tools. All patient data included in the information system can be extracted for the generation of populations or subpopulations, sorted per condition required during a specific period of study, or exported to dynamic tables to allow their joint use. Besides, it is compatible with the main programs for data use and statistics, such as Excel® or SPSS®.
Logically, SiMON-VC presents limitations; from our point of view, the main one is that it does not allow to enter automatically from the pharmacotherapeutical history of the patient all their pharmacological treatment, both prescribed in the hospital setting and out of hospital, which therefore must be entered manually and requires a review (already routinely conducted) at each patient visit to the Pharmaceutical Care Unit. A constant update is also required, for example regarding the design of alerts, rules for action, or antiviral treatment contraindication.
Summing up, SiMON-VC is a pioneer information system in Spain; it is flexible, and allows to have a protocol designed by the same specialists involved in healthcare management of HCV patients. It incorporates, in a dynamic and intuitive way, all patient information required, with functions also in the setting of quality, safety, management and research; and it also encourages compliance of the strategic lines for the professional development of Hospital Pharmacists.











 texto en
texto en