Introduction
Tacrolimus is commonly used to reduce the immune response in pediatric transplant recipients in order to prevent graft rejection1. It is characterized by high inter and intraindividual variability in pharmacokinetic parameters, a narrow therapeutic range and a documented relationship between blood concentration, safety and efficacy1. Therefore, therapeutic drug monitoring is mandatory in daily practice to increase the probability of preventing graft rejection while minimizing the probability of adverse events1,2.
Children experience physiological changes during childhood, with maturational changes in their metabolic systems, affecting the pharmacokinetics and pharmacodynamic of administered drugs3. Thus, studies in children are of high importance to assess variability in exposure of tacrolimus since this population is less frequently studied during the development of drug products4,5.
Generic immunosuppressive drug products are widespread and represent a viable cost-saving tool, especially in developing countries6,7 but also in the United States of America8. This increase in the market sales of generics of immunosuppressant drugs is giving rise to controversy regarding the necessary evaluations for their approval. The main regulatory agencies require bioequivalence studies for marketing a generic drug. The fact that these pharmacokinetic studies are conducted on healthy volunteers, has given rise to concern regarding the expected efficacy and the pattern of adverse events in transplanted patients4,9,10. Specifically, there has been an intense debate about the bioavailability of different formulations in patients, including alteration of the physicochemical properties of the product according to the excipients used or even, drug-patient interactions not observed in the bioequivalence studies performed in healthy volunteers4,10. Despite the difference of opinions, generic drugs impact the clinical routine and it is important to generate scientific evidence in this regard.
Several societies have published guidelines and opinion papers on the use of generic drugs in transplantation4,5,9. A close monitoring of patients undergoing generic-to-innovator tacrolimus formulation or vice versa is suggested to ensure a similar exposure in transplant patients4,9,11. However, most of the previous reports are in adults and very limited information is available in pediatrics8,11-17.
Hence, the objective of the present study was to evaluate the dosage, blood exposure, safety and efficacy of tacrolimus in a pediatric transplant population subjected to generic substitution in their maintenance treatment.
Methods
The development and implementation of this study was approved by the Ethical Committee of Hospital of Pediatrics JP Garrahan (Protocol #670). Internal protocols (Form 1418F62) were used to assessed medical records and clinical/laboratory parameters obtained in daily routine.
Treatment and inclusion criteria
This was a retrospective, observational study conducted between April and August 2013 by the Hospital Pharmacy Department. During this period, the Pharmacy dispensed the generic tacrolimus product (Sandoz Laboratory), according to the provision of the National Program implemented by the Central National Institute for Ablation and Implantation Coordination, Ministry of Health, Argentina.
Hospitalized patients or outpatients included who were taking a stable dose of the innovator drug (Prograf®, Astellas Laboratory, Ireland) underwent conversion to the generic product (Tacrolimus Sandoz®, India), under medical and nurse supervision (Table 1). During the conversion period, the Pharmacy Department generated reliable internal records on the brand-name medication that each patient was taking and the associated dates.
The conversion from one product to the other was carried out by administering the same maintenance dose of the generic product. Later, generic dosage was adjusted according to the physician criteria in order to maintain tacrolimus trough concentrations (C0, 12 hours post-administration) within the therapeutic range established by international consensus18. More specifically, target levels for liver transplant recipients were between 5 and 8ng/ml19; 5 to 7ng/ml in kidney transplant recipients18,20 and 8 to 12ng/ml in heart transplant recipients after 3-6 months post-transplant and 5 to 10ng/ml for patients >6 months post-transplantation21. In hematopoietic stem cell (HSC) transplant recipients, the target range of concentrations was 5-15 ng/ml.
Immunosuppression treatment scheme
The immunosuppression regimen consisted of tacrolimus as the only drug or in combination with steroids (0.06-1 mg/kg/day), mycophenolate mofetil or sirolimus21,22. In HSC transplant recipients, methotrexate was used over short periods of time in the intensification regimen (10 mg/m2 for 4 days).
Quantification of tacrolimus in blood samples
Whole-blood tacrolimus concentrations were quantified by chemoluminescent microparticle microassay (Architect®, Abbott, Chicago, USA).
The assay acceptance criteria involve a set of controls (Bio-Rad Lyphochek® Whole Blood Immunosuppressant) with limit for assay imprecision (< 7% CV) and deviation from the target control values, 2SD.
External and internal specimens were routinely assessed as part of an international proficiency testing program for quality control of the analytical technique http://www.bioanalytics.co.uk.
Data collection and patient monitoring
The following demographic and laboratory parameters were obtained before and after the switch from innovator to generic tacrolimus formulation: body weight, daily dose and whole-blood tacrolimus trough concentration (C0). Liver function was analyzed according to blood concentrations of the enzymes alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Renal function was assessed according to the serum creatinine concentrations and uremia. Similarly, the hematocrit and albumin values were recorded for evaluating possible effects on the free fraction of tacrolimus23. Afterwards, the median (range) of the laboratory parameters was calculated for each study period, and compared to the normal values reported in literature for pediatric patients24.
Lastly, an evaluation was made of the treatment safety during the follow-up period, including the most frequent and severe adverse events according to our prior reports, which include: hypertension, nephrotoxicity, neurotoxicity, post-transplant lymphoproliferative disorder, hypomagnesemia and hyperglycemia22,25. In addition, efficacy was evaluated by assessment of acute rejection episodes, graft loss and death. The rejection episodes were confirmed by biopsy and classified. In particular, for liver and kidney transplant recipients, rejection was confirmed by biopsy-based diagnosis and was recorded according Banff classification22. Acute rejection and graft-versus-host disease were diagnosed using clinical criteria and biopsy, according to the National Institutes of Health consensus for HSC transplant recipients26. The rejection episodes for heart transplant recipients were analyzed according to the International Heart Transplant Society consensus21,27.
Statistical analysis
The median of tacrolimus trough levels normalized by the dose (DNL) and doses corrected by body weight were calculated before and after switch for each patient. Data was informed as a ratio between the median of innovator tacrolimus DNL and the generic tacrolimus DNL. For statistical analysis Wilcoxon matched pairs t-test was performed using Graphpad software package (GraphPad Prism v.5). A variation of the DNL innovator-to-generic ratio equal or higher than 25% was considered more than expected variability, based on previous studies on the variability of tacrolimus exposure. Intra-patient variation in pharmacokinetic parameters has been previously reported to be between 14 and 44%1,28. Therefore, 25% was considered as a general threshold defining the expected intra-patient variability in tacrolimus pharmacokinetic parameters.
Results
A total of 33 patients were identified by the Pharmacy Department to switch the tacrolimus formulation. However, only 10 patients were finally included in this study according to our inclusion criteria (Figure 1).
The median (range) of age and body weight was 11.9 years (0.8-15.8) and 47.4 kg (7.3-77.0), respectively. Five out of ten patients were male. The median time (range) taking the innovator drug was 32 days (10-140) and the follow-up after the conversion to the generic product was 28 days (10-86) (Table 2). Enrolled patients consisted of 4 (40%), 2 (20%), 2 (20%) and 2 (20%) that received a liver transplant, kidney, hematopoietic stem cell (HSC) and heart transplantation, respectively.
Table 2 Study group demographic characteristics
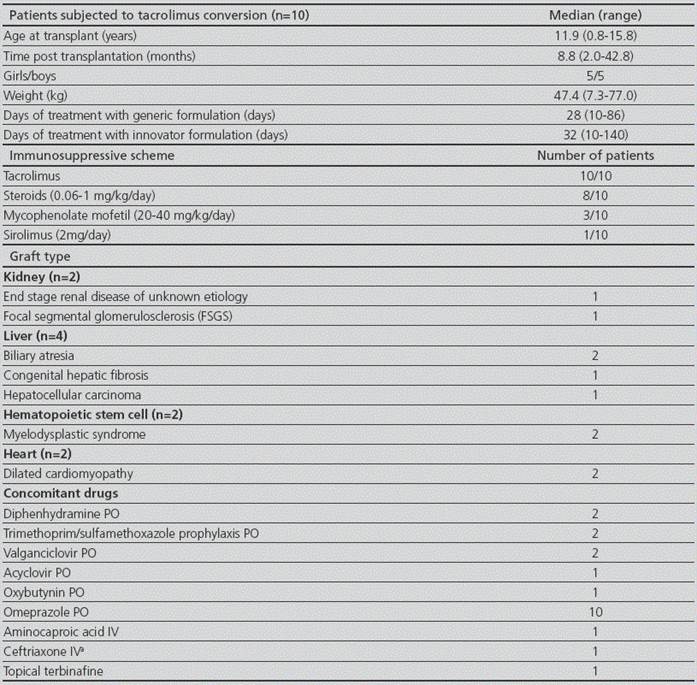
PO: oral. IV: intravenous
a: suspected respiratory infection
The median (range) daily dose corrected by body weight for the innovator formulation was 0.070 mg/kg (0.024-0.461), whereas the median for the generic formulation was 0.069 mg/kg (0.017-0.571).Thus, no significant changes were observed in tacrolimus daily dose corrected by body weight before and after conversion (p>0.05). Moreover, dose adjustments after conversion were not necessary in the study population and drug-drug interactions were not observed.
Treatment with concomitant drugs did not change during the study period except for patient #9, who received aminocaproic acid for treating a case of hemoptysis, and patient #2 that took acyclovir to treat a case of cutaneous herpes zoster.
The number of dose normalized levels (DNLs) measurements per patient, expressed as median (range) obtained with the innovator and the generic formulation was 3.0 (2.0-13.0) and 4.5 (1.0-8.0), respectively (p>0.05). Interestingly, the individual median DNL showed no significant difference when comparing between innovator and generic tacrolimus (p>0.05). Table 3 details the individual ratios between the DNLs for the innovator and generic product. The mentioned table shows that the individual values of the DNL ratios fell within the 0.75 - 1.25 range for 80% of the analyzed patients (Figure 2). Patient #8 showed a ratio of 0.72, close to the 0.75 threshold previously established based on the intra-patient variability reports on tacrolimus pharmacokinetics. On the contrary, patient #9 showed a ratio of 1.5, (mean C0 innovator/ mean C0 generic=1.5) which would indicate that the mean C0 achieved post-innovator administration was higher than that for the generic product for the same dosage.
Table 3 Tacrolimus dose normalized levels with innovator and generic formulation of tacrolimus

Data are shown as median (range) of the dose normalized tacrolimus levels with the innovator and the generic formulation. aOnly one trough level obtained during this period
DNC: Dose-normalized whole-blood tacrolimus concentration or C0/dose. I: Innovator drug. G: generic drug. Renal transplant patients: 1, 2: Liver transplant patient: 3, 4, 5, 6; Hematopoietic stem cell transplant patient: 7, 8; Heart transplant patient: 9, 10.

Figure 2 Dose-normalized whole blood trough concentrations: Ratio between the medians of the innovative and generic formulation in pediatric transplant patients.
Regarding laboratory parameters, values including serum creatinine, uremia, AST, ALT and ALP showed no significant differences when comparing the status before and after the switch (p>0.05; Figure 3 A, B, C, D, E, respectively). Moreover, hematocrit and albumin values did not show significant differences before and after substitution (Figure 2, F and G; p>0.05). Only patients #5 and #3 showed elevated liver enzyme values, but these augmented values were found in both study periods.

Figure 3 Evaluation of the individual laboratory parameters before and after conversion from one commercial brand of tacrolimus to another.
Lastly, no adverse events occurred during the period immediately before or after the substitution from innovator to generic formulation. Acute rejection episodes, graft loss or death were not observed during the studied period.
Discussion
In the present cohort of maintenance pediatric transplant recipients, tacrolimus substitution from the innovator to the generic formulation was successfully performed under close monitoring of the clinical team at a national pediatric hospital in Argentina. We showed no significant difference in tacrolimus C0 normalized by the dose before and after switch. In addition, laboratory parameters did not change after conversion (p>0.05). Adverse events, acute rejection, death and graft loss were not observed.
The National Organ Procurement Program in Argentina supplies, free-of-charge, to those patients who are in need, with the immunosuppressant drug product depending on the winning bid established by the government. Two brands of tacrolimus are currently marketed in Argentina: the innovator (Prograf®, Astellas Ireland) and the generic (Tacrolimus Sandoz®, India). The institutions taking part in the National Organ Procurement Program are supplied with and dispense one product or the other depending on this bidding procedure. This program is under Argentine health authority regulation (ANMAT, National Medicine, Food and Medical Technology Administration, www.anmat.gov.ar), which requires that the pharmaceutical companies who apply for the registration of certain products, including those containing immunosuppressive drugs, demonstrate both their in vitro (dissolution profiles) and in vivo (bioequivalence studies) quality.
The studied formulations included in the present report are bioequivalent and pharmaceutically equivalent drug products. The generic formulation that our patients received showed a comparable pharmacokinetic profile with the innovator product, including area under the concentration versus time curve (AUC), Cmax and C0,as described in previous studies in adult kidney transplant patients11. Therefore, we expected to find a similarity in the systemic exposure to tacrolimus between innovator and generic formulations.
Our findings suggest that the lack of difference between the C0 normalized by dose before and after tacrolimus substitution was in correspondence with the absence of dosage modifications after conversion in order to keep the blood concentrations within the tacrolimus target range. A total of 8 out of the 10 patients analyzed showed a tacrolimus individual DNL ratio less than 1.25 or greater than 0.75, which is in accordance with the expected intra-patient variability reported for tacrolimus28. Although it would be desirable that the ratio between DNL with innovator and generic formulations was 1, intra-patient pharmacokinetic variability of tacrolimus affects this ratio. This variability must be taken into account to avoid erroneous attributions of variability to one commercial product. The drug per se presents systemic exposure variability in one same patient, evaluated repeatedly. Therefore, the variability in the ratios of the normalized concentrations of up to 25% was assumed as an expected acceptable value according to prior reports18,28. It must be pointed out that one patient showed a variation greater than 25% (#9), secondary to an episode of hemoptysis simultaneously with the generic conversion. Hence, the existence of a hemodynamic change in that specific patient may have impacted on tacrolimus pharmacokinetic variability.
Our results are similar to those reported in the only study conducted on pediatric patients, more specifically on heart transplant patients8. In that study, tacrolimus C0 concentrations before and after the conversion from the original to the generic formulation of tacrolimus were evaluated retrospectively. In total, 12 patients who were switched from the innovator to the generic product and 31 patients who took only the original tacrolimus as a control group were included in the analysis. In the first cohort, the authors reported a 14% reduction in the C0 (equivalent to 1.15 ng/ml) after the substitution, without modification of tacrolimus dosage8. The clinical implication of a 14% modification in the C0 values is probably not important. The authors reported that after one year of follow-up post-conversion, 24% of the patients who switched and 18% of the patients in the control group showed acute rejection episodes without significant differences between groups. However, safety and efficacy parameters, as well as tacrolimus systemic exposure parameters should be studied in a larger cohort of patients in the context of tacrolimus generic substitution. According to our understanding, it is important to evaluate and describe the results not only for the population but rather the individual modification of the C0 during the switch from one brand to the other, which may be disguised by the populations analysis stated as the average values. In our study, only one patient (#9) showed a difference higher than 25% in the dose-normalized C0 after conversion to the generic product supporting the role of therapeutic drug monitoring mainly in situations which may result in an inappropriate drug exposure.
The experience reported in adult solid organ transplant patients subjected to immunosuppressants conversion is, in certain aspects, a source of controversy. The study reported by Spence et al, described the experience obtained from the retrospective evaluation of 234 adult kidney, liver and heart transplant patients in their maintenance immunosuppressive therapy subjected to conversion from one original commercial brand containing tacrolimus to the generic product13. The authors reported no significant difference in tacrolimus exposure after the substitution from the innovator to the same generic formulation that we used in our study13. It is important to point out that 15% of the patients required a dose adjustment in the context of therapeutic drug monitoring. Regarding safety and efficacy aspects of the substitution, no death or acute rejection episodes were recorded after conversion. In line with the mentioned findings, McDevvitt-Potter et al. reported their experience in 70 adult renal, liver and multiple organ transplant patients, who were studied retrospectively to evaluate dose modifications, systemic drug exposure and costs of the substitution from the original to the generic formulation12. The authors showed no significant changes in the tacrolimus daily dose or in the C0 after the switch to the Sandoz generic product12. Nevertheless, 21% of the patients subjected to substitution required dose adjustments to reach the target levels after conversion, while it was necessary in 7% of the patients assigned to the control group that only received the innovator product. The difference observed between groups of patients was statistically significant. Once again, this reveals the importance of therapeutic monitoring of tacrolimus in transplant recipients.
On the contrary, studies reported by Momper et al. and Marfo et al. conducted in adult kidney and/or liver transplant patients showed a significant difference in tacrolimus C0 values before and after the conversion from the original to the generic formulation13,17. Specifically, in the study reported by Momper et al. the authorsdescribed a 15.9% and 11.9% decrease in the dose-adjusted tacrolimus C0 after conversion in adult liver and kidney transplant recipients, respectively. Even in a subgrooup of liver and kidney transplant recipients who took the same dosage before and after the conversion, they showed an average decrase in the tacrolimus C0 of 1.98 ng/ml and 0.87 ng/ml, respectively. However, this modification in the immunosuppressive drug exposure did not show any clinical relevance regarding safety and efficacy as previously described in pediatric patients. In this sense, the retrospective study conducted by Marfo et al. showed a difference of 0.8ng/ml in tacrolimus C0 before and afterconversion to the generic formulation compared to the 0.9 ng/ml in the control group17. Likewise, despite the mentioned differences in the C0, no difference was detected in the efficacy and safety of generic tacrolimus in comparison to the innovator product. Overall, the prior reports, mainly in adults, show different types of conclusions. However, all of the authors emphasize the need of therapeutic drug monitoring of tacrolimus in patients under conversion from innovator to generic product, in order to deal with a rational scheme and assure efficacy and safety individually.
In the present article, we evaluated renal and liver function as part of the safety assessment by means of the evaluation of biochemical parameters. Specifically, liver enzyme as well as serum creatinine values did not change after conversion (p>0.05). Specifically, two liver transplant patients (#3 and #5, Fig. 3) presented stable elevated ALP, GGT, AST and ALT due to a CMV infection and gallbladder drainage, respectively. Furthermore, plasma albumin and hematocrit values were measured, since they have an influence on tacrolimus pharmacokinetics. Tacrolimus binds ~99% to the plasma proteins and erythrocytes1. Hence, a low albumin or hematocrit value leads to a greater free tacrolimus fraction available to be distributed, metabolized and eliminated. These factors, that may influence tacrolimus pharmacokinetics during the conversion process, could be a confounding variable in the evaluation of tacrolimus exposure1. Nevertheless, in our population, the hematocrit and albumin values did not show significant differences before and after substitution (p>0.05). It should be noted that our results are in line with those previously reported in pediatric and adult heart, kidney, liver and multiple organ transplant patients, in whom no significant differences were found in laboratory parameters8,13,14,17.
Based on our own published data we here evaluated the most frequent and severe adverse events to tacrolimus in the present study pediatric population were evaluated22. During the follow-up period of this study, no adverse events, graft loss, acute rejection episodes or death were observed. Therefore, we consider that the process of conversion from the original tacrolimus to the generic product was safe for the study population. Nonetheless, we emphasize the need of validating our results in a larger cohort of patients.
Argentina’s health system includes both the private and public sectors. Approximately 20% of the solid organ transplant patients take immunosuppressive drugs provi ded by their private insurance, whilst 80% are covered by the national government (INCUCAI, National Institute Coordinator Center for Organ Procurement of Argentina). Therefore, the immunosuppressive product which the majority of Argentinean patients receive depends on the winning bid established by the government. In view of this situation of generic substitution, different actions can be taken for assuring a safe conversion process. In this regard, international recommendations suggest close therapeutic monitoring of immunosuppressive drugs during the switch from one pharmaceutical product to another4,9. For such a purpose, the tacrolimus C0 values, as a practical indicator of the systemic exposure, must be closely monitored immediately before and after substitution. Laboratory parameters must be monitored as safety markers4,9. In view of the apparently inevitable conversion from one brand to another in our country, our group decided to carry out a safe and effective conversion process following international recommendations, which is presented in this report.
Our study has certain limitations. The exploratory analysis was based on a retrospective, descriptive design. Furthermore, this analysis was made on a small cohort of transplant patients. Unlike reports which describes only one type of transplantation, our study includes pediatric liver, heart, kidney and HSC transplant patients. Each patient was his or her own control, and we showed that therapeutic drug monitoring is a necessary and useful tool. Secondly, this study used the C0 values for evaluating tacrolimus exposure, although the best exposure parameter is the area under the concentration versus time curve (AUC)29. A linear correlation exists between the AUC and the tacrolimus C0 concentration, also reported for the generic product used in our study30. Lastly, the follow-up of the patients which were switched to the generic formulation was short. Hence, further studies in larger groups and with a longer-term follow-up could describe additional data of adverse events and acute allograft rejection, which may take months to manifest clinically in the setting of sub therapeutic immunosuppression.
In summary, we performed tacrolimus therapeutic monitoring according to international recommendations in a pediatric population subjected to conversion between the innovator and the generic tacrolimus formulation commercialized in the local market. We can conclude that substitution was safe and did not interfere with the efficacy of the immunosuppressant treatment in our population during the follow-up period, as there were no differences in dosage, exposure and clinical outcomes between analyzed periods. We do advocate that large prospective pediatric trials must be conducted in this field. Nonetheless, we recommend performing therapeutic drug monitoring in pediatric transplant patients undergoing immunosuppressant substitution to ensure safety and efficacy of the immunosuppressive treatment.











 texto en
texto en 




