Introduction
Community-acquired pneumonia (CAP) is associated with high morbidity and mortality rates often giving rise to hospital admissions, especially among elderly patients1. In the “Global Burden of Disease Study”, the pneumonias within the context of lower respiratory tract infections were found to be the fourth-ranked cause of global mortality and the second-ranked cause of loss of potential years of life2. In a review conducted for the purpose of determining the incidence rate and the risk factors regarding CAP being acquired by adults in Europe, the major comorbidities found to exist included chronic respiratory, cardiovascular and cerebrovascular diseases, Parkinson’s disease, epilepsy, dementia, dysphagia, HIV infection or chronic kidney or liver disease, which double or quadruple the probability of acquiring this disease3.
The CAP incidence in Europe rose from 1.07-1.20 to 1.54-1.70 cases per 1,000 persons per year³. A study conducted in the area of Tarragona involving the participation of 3 hospitals (Joan XXIII, Santa Tecla and Pius Hospital) and 8 primary care centers (Tarragona-Valls, Bonavista-La Canonja, Torreforta-La Granja, Sant Pere i Sant Pau, Tarraco, Sant Salvador, Salou and Valls) included 11,240 persons age 65 or older and showed the CAP incidence to increase significantly with age, having totaled an incidence of 9.90 cases per 1,000 persons per year within the 65-74 age range as compared to the 29.40 cases per 1,000 persons per year in persons over 85 years of age (p < 0,001)4.
Nosocomial pneumonia is one of the most frequent infections in critically-ill patients5. From the etiological standpoint, methicillin-resistant Staphylococcus aureus (MRSA) is one of the most prevalent microorganisms in this infection and may be found in 27% of the critically-ill patients who have this infectious disease6. Despite methicillin-resistant Staphylococcus aureus (MRSA) having often been associated with nosocomial pneumonia in patients admitted to ICU, MRSA CAP in Europe is estimated to fall within the range of 0.51-0.64 cases per 100,000 inhabitants, entailing a mortality rate of 10%-25% in patients requiring hospitalization7.
In order to select the best option for treating MRSA community-acquired pneumonia, it is indispensable to know both the clinical characteristics of the patients and the pharmacokinetic variations which have a bearing on the concentrations of the antibiotics used in their treatment, as well as their sensitivity to MRSA. The purpose of this review is to conduct a systematic review of the literature on the antibiotic treatment of MRSA CAP in critically-ill patients.
Material and methods
A thorough online search was conducted of the bibliography published on MRSA CAP in critically-ill patients. The relevant publications were identified in PUBMED, the BestPractice database, UpToDate database and the Cochrane Plus Library for articles published in English within the December 2001 - April 2016 time frame. The key words used for this search were: “pneumonia” and “critically ill” or “intensive care unit” and “methicillin resistant staphylococcus aureus” and “community-acquired”. On the basis of the articles found in the search, a manual search was conducted in the reference lists for the purpose of identifying the relevant articles.
Figure 1 includes meta-analyses, systematic reviews and revisions, clinical trials, observational studies, clinical practice manuals, consensus conferences and abstracts in English. Those articles on in-vitro efficacy, animal model studies, pediatric studies and those not including a diagnosis of CAP or whenever the article included CAP of a different etiology and did not make a specific analysis of the results of the MRSA CAP cases, termed as being non-specific in approach, were excluded. Two independent reviewers took part in the process of selecting the articles and, in view of any discrepancy, a third senior reviewer having made the selection.
Results
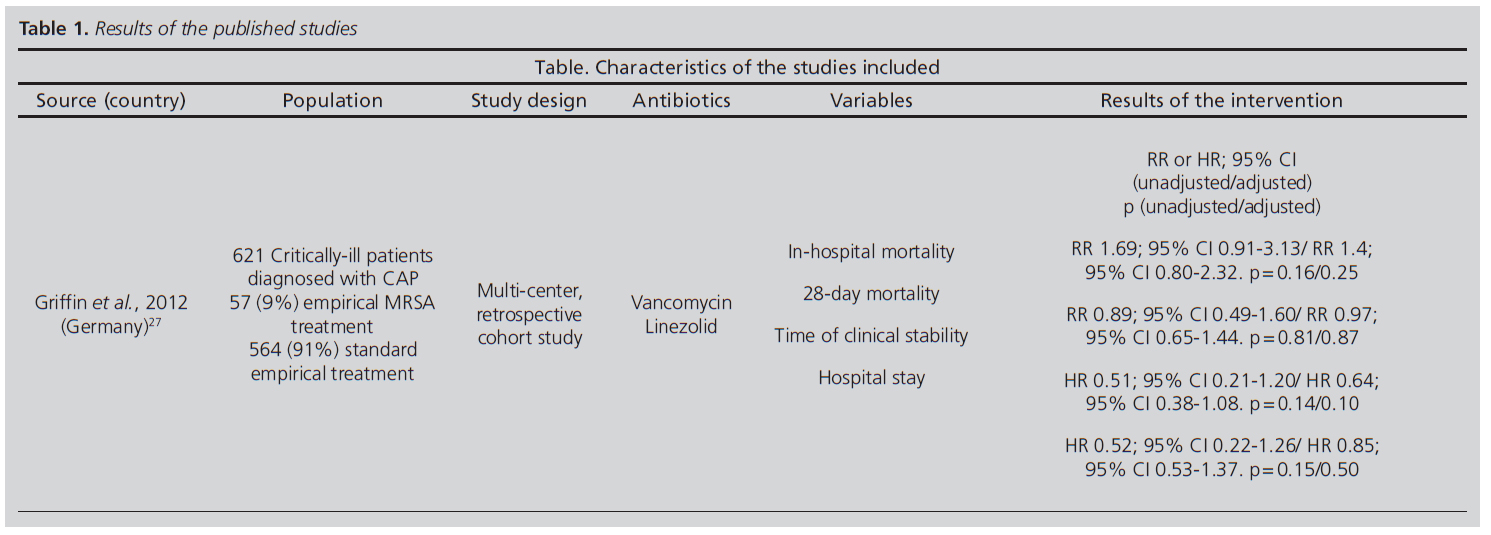
A total of 70 potentially-relevant articles were identified. After having conducted a complete review of the texts, a total of 13 studies (18.84%) met the requirements for inclusion, a total of 57 (81.4%) having been excluded (Table 1). The cohort studies were predominant, having totaled six in number (20.69%) as compared to one sole article published on a cross-sectional study (3.45%).
Table 1 Results of the published studies




CA-MRSA: Community-acquired methicillin-resistant Staphylococcus aureus
RR: Relative Risk
HR: Hazard Radio
OR: Odds Ratio
CI: Confidence Interval
CAP: Community-acquired pneumonia
HAP: Hospital-acquired pneumonia
ICU: Intensive Care Unit
MRSA: Methicillin-resistant Staphylococcus aureus
MSSA: Methicillin-sensitive Staphylococcus aureus
HCAP: Healthcare-associated pneumonia
PVL: Panton-Valentine Leukocidin
Discussion
The information available on the treatment of MRSA community-acquired pneumonia in patients requiring admission to ICU is quite limited. This paucity of information could be due to the studies having focused mainly on nosocomial pneumonias. However, this disease shows itself to be significantly relevant in clinical practice, given that it is associated with a high mortality rate8.
In the present systematic review, the predominant treatment in this infectious entity was found to be vancomycin. In addition thereto, as a major limitation, the studies have focused on populations affected by different etiological agents and not solely on MRSA, which totaled only a minor percentage.
Vancomycin has been the treatment of choice in recent years. However, the difficulty of achieving therapeutic levels at the pulmonary level, in conjunction with the current controversy concerning the influence of the minimum inhibitory concentration (MIC) on the efficacy of vancomycin has led to other treatment alternatives being sought9. In this regard, MRSA strains with a MIC for vancomycin ≥ 1.5 mcg/ml have been found to be associated with worse clinical outcomes in different types of infections10.
However, one must bear in mind that none of the studies analyzed specifically deal with the impact of higher vancomycin MICs on the prognosis of MRSA CAP patients, unlike what has been studied in MRSA nosocomial pneumonia11.
To deal with all of these limitations, the combination of rifampicin and vancomycin has recently been evaluated in one review, showing a possible benefit on the treatment of MRSA pneumonia, although it has not as yet been specifically evaluated in MRSA CAP11.
Linezolid is an antibiotic which has widely demonstrated its efficacy in treating pneumonia9, although no specific experience in MRSA CAP currently be available. However, given its ability to reach high concentrations in the pulmonary epithelial lining fluid12,13, it could be an effective alternative for the treatment of MRSA community-acquired pneumonia. Additionally, its use could be especially indicated in patients diagnosed with MRSA CAP who produce Panton-Valentine Leukocidin (PVL), taking into account the ability of linezolid to inhibit the production of this toxin14,15. Another of the advantages of using linezolid is its excellent pharmacokinetics, making a sequenced treatment from the intravenous route to the oral route possible due to its 100% bioavailability16.
Other antibiotics available on the market for treating MRSA CAP are telavancin, ceftobiprole (neither of which are not marketed in Spain), ceftaroline, clindamycin and cotrimoxazole. Telavancin has not been evaluated for MRSA CAP, but rather for the treatment of the hospital-acquired pneumonia (HAP) caused by this microorganism17. As far as the two cephalosporins are concerned, the only published studies available provide a low degree of scientific evidence, such cohort studies including a small number of patients or case series. In the case of the use of ceftobiprole, a clinical study is available comparing it to ceftriaxone, making it possible to add linezolid to this branch in view of clinical signs and symptoms of suspected MRSA18. This study revealed ceftobiprole not to be inferior to the drug with which it was compared. As far as the use of ceftaroline in CAP, there are only two clinical trials available, but they excluded patients admitted to the ICU in whom MRSA infection was confirmed.
The use of clindamycin is described only to a minor degree in patients diagnosed with MRSA CAP. However, the use of clindamycin associated with linezolid provided good results in comparison to the vancomycin and rifampicin combination, given its ability to block PVL in the case of a critically-ill patient diagnosed with MRSA CAP.15.
As far as cotrimoxazole is concerned, just as in the case of clindamycin, there is little evidence as to its use in MRSA CAP. Bearing in mind the major activity of both of these antimicrobials combatting out-of-hospital MRSA strains, as well as their easy handling on being possible to administer orally or intravenously, they theoretically seem to be treatment alternatives of interest for the treatment of MRSA CAP19. However, it is advisable to stress the absence of clinical evidence of these antibiotics for this indication.
Critically-ill patients show widely-varying pharmacokinetics regarding antibiotics20. Multiple organ failure, increased volume of distribution (hypoalbuminemia, resuscitation fluids, shock, edemas...), as well as the use of vasoactive drugs and extracorporeal circuits can give rise to a high degree of variability in the plasma concentrations20. As a result of all of the foregoing, different treatment strategies have been studied in an attempt to achieve a greater stability in the plasma concentrations, such as the administration of antibiotics in continuous infusion for those of time-dependent bactericidal activity.
This high degree of variability in the plasma concentrations gives rise to a great deal of uncertainty regarding the final quantity of antimicrobial reaching the site of infection, the lung in this case. In this regard, more selective administration routes have been studied, making it possible to achieve a higher concentration at the site of infection with less of a risk of adverse systemic effects. One such technique is the administration of aerosolized antibiotics.
In the present review, all of the studies analyzed the different treatments for MRSA CAP when the antimicrobials were administered as in standard practice, that is to say, in intermittent intravenous infusion. However, there are alternative treatment strategies, given that both linezolid and vancomycin could be administered in continuous infusion, and other administration routes could be used, such as via aerosolized administration, although there is no information of MRSA CAP.
Continuous infusion of vancomycin has not shown itself to provide efficacy-related benefits in comparison to intermittent administration in patients with different diseases, although it apparently might cause a lesser degree of nephrotoxicity22. However, its potential benefit in MRSA CAP is unknown, given that it has not been studied specifically.
In the case of linezolid, limited information exists on its administration in continuous infusion, said information having to do mainly with HAP23. A clinical case was presented recently in the form of a poster and oral presentation involving a critically-ill patient diagnosed with MRSA necrotizing pneumonia. After having initially undergone treaty with vancomycin, given the resulting poor evolution, linezolid in continuous infusion was started at higher than standard dosages of 600 mg/12 h, which enable the patient to show a proper evolution with clinical cure24.
Another more innovative strategy with a promising future lies in the administration of certain aerosolized antibiotics, given that the concentrations reached in the pulmonary lining fluid can be of less than 50% of those detected via plasma25. One of the possible tools for remedying this problem would be the use of aerosolized antibiotics, the use of which is scarcely repor ted in the literature. This administration route makes it possible to achieve local levels much higher than the serum levels (by following a suitable technique, the levels could be increased by up to 650%)25, with a lower systemic concentration of antibiotic and hence a lesser risk of adverse effects26.
Conclusions
Myriad active molecules are available for combating MRSA which have been used in the treatment of pneumonia, although the experience in patients with MRSA CAP requiring admission to ICU is quite limited. Especially in critically-ill patients, vancomycin or linezolid are apparently the treatments of choice for treating MRSA CAP, although there not be any specific recommendation in this regard. The clinical pharmacist may be useful in recommending and providing advice concerning alternative strategies for administering certain specific antibiotics, such as linezolid or vancomycin, both of which can be administered in continuous infusion or in association with other antibiotics. Clinical pharmacists may also be useful in assessing alternative routes, such as aerosolized, all of which are options with a promising future in treating this disease.
However, the clinical efficacy of these treatment strategies has not been evaluated in MRSA CAP, thus substantiating the need of conducting future studies on this infectious disease with a high morbidity and mortality rate.











 text in
text in 



