Introduction
There have been concerns about the safety of handling medications with risk for the healthcare staff since Falk published on 1979 an article about the detection of mutagenicity in the urine of nurses preparing cytostatic drugs1. Nowadays, this is still an area of concern in the hospitals, mainly at pharmacy level, as well as for the Occupational Risk Prevention Departments. The preparation of cytostatic drugs in centralized and specialized units has become a regular and established practice, based on the guidelines for adequate and safe handling of hazardous drugs2,3.
The concept of occupational exposure appeared by the first time at the end of the 70s, and it referred specifically to antineoplastic medications. Some years later, at the beginning of the 80s, an increasing concern about potential risks led to The Occupational Safety and Health Administration (OSHA) to publish guidelines on handling cytotoxic compounds5. Currently, other agencies such as
The American Society of Hospital Pharmacists (ASHP) or The National Institute for Occupational Safety and Health (NIOSH) would periodically suggest new improvements on this area.
The term Hazardous Drug was introduced by the ASHP in 19904 and adopted by the OSHA5 for those medications which presented at least one of the following risk characteristics for humans: carcinogenicity, teratogenicity or any other toxicity for the development, reproductive toxicity, organ toxicity at low doses, genotoxicity, or those drugs with a similar structure or toxicity profile to other hazardous medications.
Along 2004, the NIOSH published a list of hazardous drugs6, which was updated in 2010 7, 20128 and 20149; the 2016 version, is the last one, and it is currently available10. Based on the 2014 publication, the NIOSH classified hazardous drugs into three groups: Group 1: antineoplastic drugs, Group 2: non-antineoplastic drugs that present at least one criterion for being hazardous; and Group 3: those drugs that present risk for the reproductive process, and that might affect men and women who are actively trying to conceive, and pregnant or breastfeeding women, but that present no risk for the rest of the staff.
Since the 2014 update, the NIOSH introduced List 2 of non-antineoplastic hazardous drugs, which forced a review of their handling in every healthcare institution, and particularly, of the duties at Hospital Pharmacy Services.
The objective of this article is to describe the actions taken by the Pharmacy Service in a tertiary hospital, in order to follow the recommendations established by NIOSH 2014 for handling hazardous drugs.
Methods
A retrospective observational study was conducted based on the recommendations done by NIOSH 2014 at tertiary hospital level, including those ones mentioned in the 2016 version (which was still a draft at the moment of the review).
The identification of the measures adopted was sequentially conducted over time, assessing the different kind of changes, which had been required such as informative, organizational and process management. Medicines available at the hospital were reviewed, and different actions were taken accordingly: replacement of drugs with a more adequate pharmaceutical formulation, selection of drugs which avoid handling or repackaging, classification of drugs that have to be prepared outside the wards, and generation of information on proper handling of hazardous drugs by healthcare staff.
Finally, in order to evaluate the measures to be adopted, there was a review of the processes for acquisition, repackaging, preparation, circuits, organization, dispensing and identification.
Results
Informative, organizational and management measures have been adopted at the Pharmacy Service, following the next steps:
Preparation of the list of hazardous drugs in the hospital and recommendations for its use
A review was conducted on the medications available at hospital that were included in any of the groups by NIOSH 2014 and by the 2016 draft available at the time of the study; drugs marketed in Spain, foreign medications, and those used in clinical trials were considered. The list identified 134 molecules, including pharmaceutical formulations used at hospital, and the recommendation for their preparation and administration was described.
This list was presented to the Hospital Management, Medical Management, Nursing Management, and Occupational Risk Prevention Department.
Assessment of Hospital Management´s needs
A report on the identified needs for Hospital Management was prepared. The following functional needs were indicated: a new clean room for the preparation of sterile non-antineoplastic hazardous drugs, availability of individual protection equipment in the hospitalization wards, and availability of nursing staff for an expanded preparation of medications in the Pharmacy Service. Apart of describing the identified needs, this report also aimed to ensure awareness by the Hospital Management and support the legal coverage of the Pharmacy Service.
Although there were clean rooms in the Pharmacy Service for the preparation of antineoplastic drugs, these were located at the “Daily Hospital”, which is very far from the General Pharmacy. The new recommendations for handling non-antineoplastic hazardous drugs represents an interruption in the antineoplastic routine preparation, and therefore it was considered necessary to request the installation of a new clean room in the General Pharmacy, where the future preparation of non-antineoplastic hazardous drugs could be performed without interfering in the scheduled activity of the Oncohaematology Daily Hospital.
Medication Prescription and Administration electronic system: integration of new recommendations
The Electronic Prescription System (Millenium®) was updated to indicate the risk of handling during the preparation and administration of all hazardous drugs in Lists 1, 2 and 3, by incorporating the following text:
Lists 1 and 2: “Risk for the Healthcare Staff: Please follow recommendations for administration”.
List 3: “Reproductive Risk for the Healthcare Staff: Please follow recommendations for administration”.
On the other hand, all those medications in Lists 1 and 2 (76 molecules in total) were marked as “Don’t Split” in the Electronic Prescription Program, so in case there was a need of splitting the medication, and alert would appear in the pharmaceutical validation section, warning about conducting it in the Pharmacy Service, and more specifically in a Biological Safety Cabinet (BSC) if necessary. However if there were alternatives as oral suspension, syrup, or oral drops, was also indicated, in order to avoid tablet splitting.
Acquisition of Medications
The review of hazardous molecules led to some changes in the acquisition of medications. Low-dose formulations of the same drug were incorporated, in order to avoid dose splitting. To avoid oral powder formulations, it was considered to replace them by oral tablet formulations. For example, megestrol was available as powder sachets (Borea), which involved dissolving the powder for administration; Megefren, which presented tablets as single doses, replaced this. Those formulations that were presented as bottles of multipledoses such as fenitoine (Epanutin) were eliminated and replaced by blisters (Sinergina).
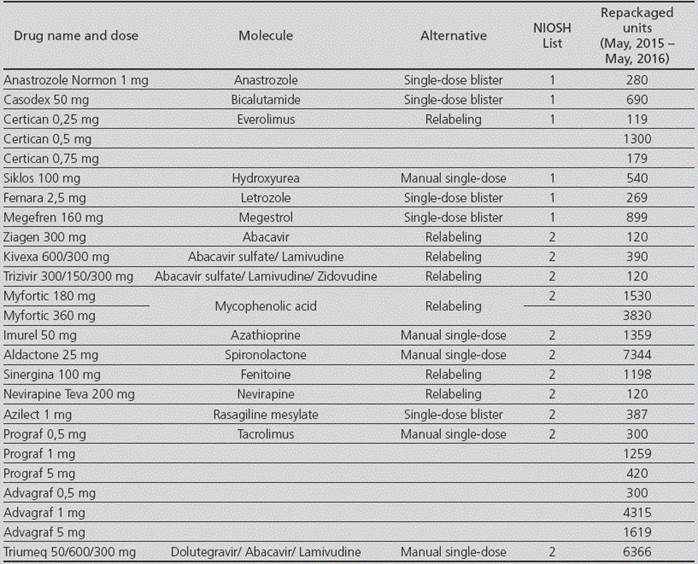
Repackaging of oral solid drugs
There was a review of all repackaging conducted in the period between May, 2015 and May, 2016, which showed that 75,779 units of hazardous drugs had been repackaged (4,290 from List 1, 36,090 from List 2, and 35,399 from List 3), being a 13.5% of the total. Out of the total drugs repacked, there were 198 (0.26%), which presented incidences in the repackaging machine.
For the molecules included in Lists 1 and 2, there was a review of the availability in the market of medications that did not require repackaging, and a single-dose formulation was found in 5 cases: anastrozole, bicalutamide, letrozole, megestrol and rasagiline.
In those cases,where drugs were not available as single-dose in the market, there was an assessment of how many were adequate for relabeling instead of repackaging; this was possible for 6 medications: everolimus, abacavir, fenitoine, nevirapine, abacavir/lamivudine and mycophenolic acid.
For the rest of the drugs without single-dose formulation, or for those that did not meet the criteria for relabeling, it was established that they had to be prepared through a manual procedure, in order to avoid using the automatic repackaging machine. Detailed information about handling was included in its repackaging specifications. These medications were: hydroxyurea, azathioprine, tacrolimus and dolutegravir/abacavir/lamivudine.
These measures solved the automatic repackaging for 35,253 units: 8,906 were relabeled, 23,822 were sent for manual preparation, and for the remaining 2,525, a marketed single-dose alternative was found, which prevented direct contact with the hazardous drug (Table 1).
Additionally, repackaging specifications for molecules in List 3, were also included to ensure the safe handling by the hospital staff, by indicating their classification as hazardous drugs, by warning to avoid the automatic repackage, and by requesting the need of exclusive-use equipment for protection of the staff, such as gloves, protective lab coat and mask, against any potential breakage or spillage during repackaging.
Preparation of non-sterile medications and oral magistral formulations, and identification of hazardous molecules
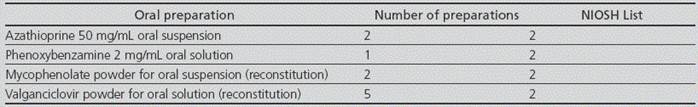
The reconstitution of oral suspensions that were marketed as powder for suspension was taken over for preparation by the Pharmacotechnology Lab: valganciclovir oral suspension, and mycophenolate oral suspension. On the other hand, recommendations for hazardous drugs were included in the preparation specifications for azathioprine 50 mg/mL oral suspension and phenoxy-benzamine 2 mg/mL oral solution. These 4 preparations were now conducted in a class IIb BSC for non-sterile formulations, located in an isolated room with ante-room, close to the Pharmacotechnology Lab, with neutral air pressure and not requiring sterile conditions. Detailed instructions were included in the relevant specifications about the safe preparation of these drugs. The number of preparations conducted for each medication is detailed in Table 2.
Preparation of Sterile Medications
The preparation of all those parenteral formulations of medications included in List 1 and 2, which were previously prepared at the ward by the nurses, was now indicated to be conducted at the Pharmacy Service in a class II b BSC.
It was detected that 3 medications for parenteral administration that required preparation in a BSC had not been prepared so far in this way; these drugs were mycophenolate, cyclosporine and tacrolimus. The preparations conducted are detailed in Table 3.
Table 3 Incorporations and changes in endovenous preparations conducted at Pharmacy Service (September, 2015 - June, 2016).

BSC IIb: Biological Safety Cabinet Type IIb HLFC: Horizontal Laminar Flow Cabinet
In case of phenytoin, it was considered purchasing it as vials through the community pharmacy, although its short self-life of 3 months did not make possible its use at hospital. Moreover, this medicine is commonly available in the trolleys for the emergencies, thus the Pharmacy Service cannot take responsibility on its preparation for emergency cases. In some cases, it has been recommended to replace phenytoin with other medication for preventing convulsions. On the other hand, even its use is not very frequent according to the recommendations by the NIOSH, eye and respiratory protection must be used, in case it cannot be prepared in a safety cabinet, because closed drug transfer systems cannot be used given its formulation as ampoules, similarly to what happens with cyclosporine and tacrolimus.
Organization for Preparation of Magistral Formulations
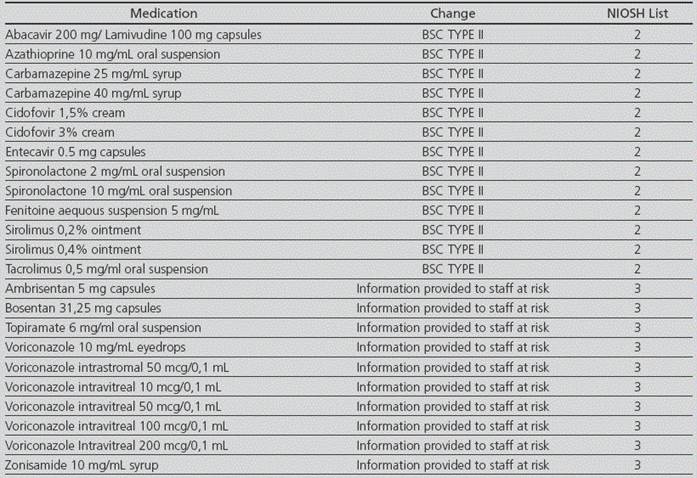
There was a review of the procedures involving hazardous drugs in the Pharmacotechnology Lab, in order to adopt safety measures for the staff. For the molecules from NIOSH List 1, there was no detection of any new activities to be conducted, because these drugs were already prepared in BSCs. For the drugs from NIOSH List 2, a section was included regarding safety rules for preparation by indicating the personal safety equipment required (blue uniform, blue clogs, cap, protective goggles, lab coat protecting against cytostatics, FFP3 mask, double gloves), and the setting of preparation (BSC). Regarding to the drugs from NIOSH List 3, the procedure was updated in order to provide information to the staff that is actually at risk (“risk for the male and female members of staff who are actively trying to conceive, women who are pregnant or planning to be, or breastfeeding women. If at a risk situation, please work in a BSC and with personal safety equipment”) (Table 4).
Discussion
The Pharmacy Service of the hospital where this study has been conducted has an ISO 9001 Rule Accreditation, and has been authorized by the Regional Health Administration to prepare oral cytostatic agents according to the Guidelines for Good Practice on Preparation of Medications in Hospital Units11.Even so, the publication of the NIOSH 2014 recommendations involved a major change in handling medications, both by the number of new medications considered hazardous, and by the change in the management of their administration, which required a re-evaluation of the processes at the Pharmacy Service and its structure.
The identification of hazardous drugs with pharmaceutical forms appropriate to prevent unsafe handling, has been a success; this applies to two drugs. On the other hand, 7.2% of the alternatives sought for repackaging avoided direct handling, due to their marketed single-dose formulation, and the remaining 92.8% improved safety in terms of contact with hazardous drugs, due to the relabeling and manual single-dose alternatives, thus avoiding incidences with the packaging machine. For the rest of the drugs, including those in List 3, detailed safety measures have been incorporated so that their handling will be as safe as possible.
Regarding to the endovenous formulations and the preparation of magistral formulations, the implementation of the NIOSH recommendations has required some processes to be corrected, thus improving the safety conditions. Specifically, some aspects have been changed in the preparation of ten hazardous drugs, which amount to the total sum of 1,257 preparations from September 2015 to June 2016; and therefore, all these contacts will be avoided in future preparations.
The qualitative improvement in terms of safety increased, is being achieved through the generation of the adequate alerts, both in the safety specifications and in the preparation specifications of the medications involved, in the Millenium® prescription program, or in the Hazardous Drugs Registry from the Health System.
Those medications that are presented as ampoules (fenitoine) is still an unsolved problem, as well as those that, due to their stability, cannot be prepared in the Pharmacy Service such as triptoreline, due to their immediate or unstable administration. It would be desirable that these hazardous drugs offered a formulation that allowed safe handling in the hospitalization wards, such as for example precharged syringes with specific safety systems.
This review is limited to the medications available at one hospital; therefore, each institution should assess the medications used in their setting and the risks in their own organization, adopting the adequate measures in each case, that will depend on their baseline situation, requiring a higher or lower number of actions, although with common objective of ensuring a safe use of the hazardous drugs in each centre.
The publication of NIOSH 2014 and its recent 2016 update12, as well as the publication in September 2016 of the technical document “Hazardous Drugs: Measures for their Preparation and Administration13”entails a higher responsibility for the Pharmacy Service. The preparation of a higher number of hazardous drugs must be centralized, incorporating an area for preparation of hazardous drugs outside the Oncohematology area, to avoid interfering with the scheduled preparations for Oncology and Hematology treatments and that presumably will require, in some cases, additional clean rooms for an adequate compliance of the new recommendations. Specific rooms for non-sterile medications should be considered as well.
Being the Pharmacy Service the main location for hazardous drug handling, force the Service to extreme the precautions, by having clean rooms with all safety guarantees required, and the use of closed drug transfer systems for the preparation of hazardous drugs.
It is necessary to guarantee safe handling, by ensuring the constant compliance of all standard procedures. It would be useful to have devices to assess and monitor the contamination in work surfaces, as well as external certifications to guarantee the safe handling of these medications.
Finally, it is worth pointing out that, although the measures to be adopted in the Pharmacy Service are essential, the safe handling of hazardous drugs affect the whole healthcare organization, and must be accepted by the whole institution in a responsible manner, because the measures taken by the Pharmacy Service will only partially avoid the total of the problem.











 text in
text in