Contribution to scientific literature
Pharmaceutical Care to HIV patients is one of the most development areas by hospital pharmacist. The impact of this activity on health outcomes has been reflected in many scientific publications.
In the last few years, number of HIV patient has increased due to the decrease in morbi-mortality associated with highly active antiretroviral therapy. In parallel, newly diagnosed patients have been incorporated into pharmacotherapeutic follow-up. Additionally, all guidelines worldwide reflect the need for universal treatment for all patients. Therefore, a great specialization is required to adapt pharmaceutical care to the needs and views of patients.
For the first time, our study support the design and adaptation of a selection and stratification model for PC in HIV+ patients to identify those who may benefit more from the intervention by the Hospital Pharmacist. Required actions and interventions for each kind of patients is also specified. It is necessary to include a comprehensive vision of Pharmaceutical Care and to involve further multidisciplinary collaboration.
Introduction
Antiretroviral therapy has led to a substantial increase in life expectancy and quality of life for HIV-infected patients, and it reduces virological transmission. As a result, current treatment guidelines recommend antiretroviral therapy for all HIV-infected individuals1,2. While drug therapy has become more convenient, HIV infection still requires lifelong treatment. As HIV infected individuals are experiencing a life expectancy close to that of the HIV-negative population, some comorbid conditions, including those associated with ageing, have become increasingly prevalent3.
Therefore, HIV-positive patients are likely to be prescribed a number of different medications both for HIV related and unrelated conditions. Such polypharmacy leads to drug interactions and overlapping toxicities, can be costly, and as medication complexity increases, may affect treatment adherence and virologic suppression3,4.
On the other hand, new infections occur in young people with a good educational level but with a low perception of risk and life implications5. These patients demand a new relationship with health professionals, including the use of new technologies.
The Hospital Pharmacist has a close relationship with these patients, therefore/so Pharmaceutical Care (PC) in this field is widespread6,7. This practice has been proved useful in improving adherence, identification, prevention and management of adverse effects and resolution of drug-related problems8. However, this activity has been traditionally performed using an individual and medicine-centered design. This individualized, patient-focused philosophy was introduced in order to address an extensive drug-induced morbidity, and poor outcomes resulting from a depersonalized healthcare system (and a drug-focused Pharmacy profession). In addition to defining a philosophy of practice, the term PC has also been used to represent a process of care that outlines the steps required to identify and resolve drug therapy problems. Other clinical processes have been introduced to help to operationalize the goal of the PC philosophy, such as medication therapy management and, more recently, comprehensive medication management. The patient care processes outlined for both include an assessment of patients’ medication needs, identification of all medication-related problems, development of a care plan, and patient follow-up to assess outcomes9.
The increasing number of HIV-positive patients and their complexity makes it necessary to develop risk classification systems to facilitate the optimization of resources and the development of the most appropriate intervention strategy for each of the levels established. Until now, there are no published classification systems from the perspective of the Hospital Pharmacy.
The aim of this study is to design a risk-stratified model for PC in HIV-positive outpatients.
Methods
A cross-sectional, multicenter study conducted between February and June 2015. An expert panel was created from a group of Hospital Pharmacist experienced in PC for HIV-positive patients from 12 Spanish hospitals belonging to the Pharmaceutical Patient Care HIV Working Group from the Spanish Society of Hospital Pharmacy.
The study was designed in 4 phases. The first phase included a review of literature and the development of a summary of the scientific evidence available at the time of the study. The values of each variable included in the model (demographics, sociographics, clinical and drug-related) were defined through a participatory approach. These variables were an adaptation from the Selection and Pharmaceutical Care for Chronic Patients Model of the Spanish Society of Hospital Pharmacists for HIV-positive patients (coinfected with HCV or not)10. Based on this review, and in coordination with some external experts in the field, we defined the relative weights of the same variables in terms of their importance to the comprehensive risk measure patient. Telephone interviews were conducted with physicians experts in the management of HIV-positive patients, in order to confirm the value and weight of the chosen variables and evaluate the inclusion of some variables with no previous consensus so far. According to the score, patients were stratified into three levels of PC, allowing the panel of experts to set parameters for each variable to be measured. We evaluated the risk of drug-related problems (DRP), the need for pharmaceutical care and the feasibility of obtaining variables.
In the second phase, a sample of 215 HIV patients from eight hospitals was assessed by Hospital pharmacist during a regular clinic appointment, through standardized data collection which included all the parameters defined in the first phase protocol. The sample size was calculated by using an estimation of 5% of patients which are regularly taken care in a week. They were selected randomly between 31 March and 16 April 2015.
In a third phase, data information was recorded for those patients in the sample. Then the parameters for each variable were redefined. Again, the results helped the expert panel to shape the items that should be evaluated in each model. The overall analysis also allowed Pharmacists to define the actions to be applied at each level of priority.
Finally, each stratification model was applied to a new sample of 205 patients to verify their applicability and usefulness (pre-test). The inclusion of patients was randomly conducted at each outpatient unit of the 8 participating hospitals.
Results
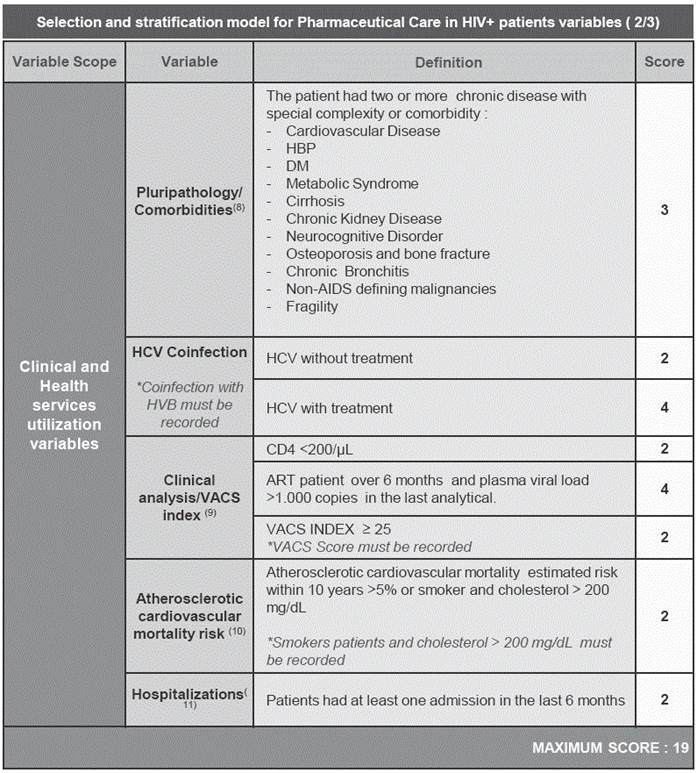
The variables finally included in the model, and their score based on their priority for pharmaceutical interventions, are summarized in Appendix 1. All variables included in the model were weighted in terms of their relative relevance compared to the rest, with a value ranging from 1 (minor relevance), 2-3 (intermediate relevance) to 4 (high relevance).
A sample of 205 patients was evaluated at the pre-test stage. Most of the patients were 30 to 50 years of age (52.7%), 8.3% had an advanced immune deficiency (CD4 below 200cell/ml) and 5.8% had a high viral load (>1000copies/mL) on stable treatment. The percentage of patients with two or more comorbidities (chronic diseases) was 25.3% and polypharmacy percentage was 31.7%. The score obtained and the distribution rate of patients in each level is shown in Figure 1.
The basis for assessing the patient according to the Model Selection of HIV+ finally agreed upon by the panel of experts and Its frequency of application is shown in Table 1. In this regard, if the patient was HIV-HCV coinfected, it was recommended that the assessment of the Selection Model in HCV patients should be done at treatment initiation, and the periodicity defined for the HIV model should be implemented afterwards.
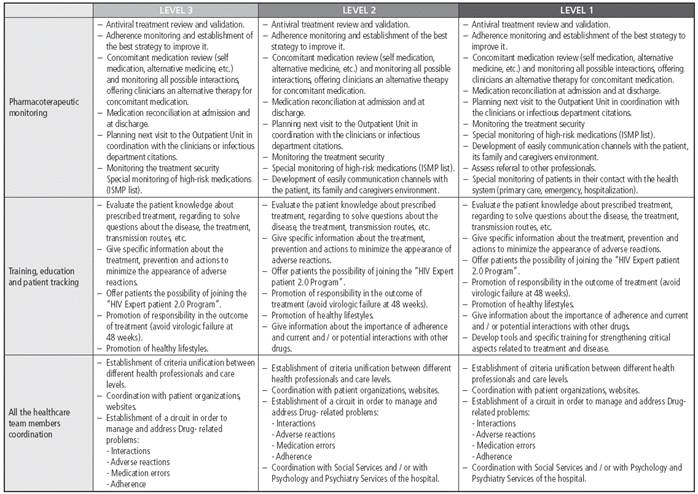
The PC interventions corresponding to each level of priority are shown in Figure 2. These actions were cumulative, so that Priority 3 patients undertook that level plus Priority 2 and Priority 1 levels.
The frequency of pharmacotherapeutical monitoring in HIV patients was recommended according to their priority level (although this will always be subject to the best judgment by each professional). Priority-1:1-2 months; Priority-2: 3-4 months; Priority-3: 6-8 months.
It was considered essential, for the optimal performance of the proposed actions, to have standard operating procedures in hospital Pharmacy Departments, to be used as guidelines for activities, to perform quality assurance and process procedures. Pharmaceutical interventions must always be registered in the patient medical records.
The standard work processes defined by the model based on possible contact situations with the Hospital Pharmacist responsible for follow-up (hospital admission, discharge, and outpatient) are shown in Table 2.
Discussion
As far as we know, this is the first model specifically designed to select and stratify HIV-positive patients for PC.
Traditionally, the Pharmacist activities have been developed based on a drug-centered model, with an episodic conception, which has prioritized the single first visit and changes in treatment; but PC improves this concept, in order to provide the responsible provision of drug therapy for the purpose of achieving definite outcomes that improve a patient’s quality of life. Several reasons could explain this drug-centered model, but we could mainly include the following: low persistence of prescribed drugs, adherence problems due to the pharmaceutical forms available a few years ago, which required complex regimens and the consequent resistance (under certain conditions), the severe and frequent occurrence of adverse effects, as well as no training and information about treatments and/or conditions for patients. However, these characteristics have been currently changed.
This definition and philosophy of PC should be modified to make it clear that Pharmacists must be responsible for those populations at high risk of drug or disease-induced morbidity. The expanded definition of PC should be understood to include a patient-centered practice in which the practitioner would be accountable for the drug-related needs of specific individuals as well as groups of patients, within a defined practice setting, who are at high risk of drug- or disease-induced morbidity.
In order to conduct PC as defined in the model, technological tools are required, to develop training initiatives for health professionals, and to define work procedures in collaboration with other health professionals and public stakeholders.
The following needs have been identified by the expert panel:
Having a standard tool for the evaluation of the validation and adequacy of treatments in the Hospital Pharmacy, such as information systems (shared electronic medical records), to see all the medication in HIV- positive patients (including those coinfected with HCV).
Having a basic tool for training Hospital Pharmacists, physicians, and nurses, about PC in HIV-positive patients.
Training of Hospital Pharmacists on questionnaires like PHQ (Patient Health Questionnaire) management, and to standardize the collection of key information for assessing the variable of mental disorders, cognitive impairment and functional dependence.
Development of focused training for Hospital Pharmacists in case management concepts and working methods to evaluate social health (functional scales and cognitive assessment, etc.).
To incorporate the model into the continuing professional development program of basic training on PC for HIV-positive patients, especially for residents. The patient-centered care processes of PC need to be expanded to include procedures that guide Pharmacists on how to perform an assessment of needs in their unique clinical practice setting, which will facilitate the process of patient selection and prioritization for PC. This assessment would be conducted in collaboration with other healthcare team members, to define disease- and medication-related priorities among their patient population.
Finally, Hospital Pharmacists would need to establish procedures to ensure that all patients included into a high-priority area would be identified to receive the best PC. Thus, there is an urgent need for defining procedures in a teamwork setting with other health professionals, within and outside the hospital, designed to improve the pharmacotherapy for HIV-positive patients. These would include the establishment of partnerships with Patient Associations to promote two-way communication between agents for the benefit of the patient, and with public and private authorities for the implementation, operation and use of data recorded in the different hospital/regional systems.
To achieve all this objectives and the PC concept proposed, it is necessary to consider not only drug-related variables, but also those related to health and social aspects, and the cognitive and functional status of the patients.
Throughout the consensus project, the expert panel and reviewers have been mindful of the high degree of variability in the health status of people living with HIV/ AIDS, and the factors that determine their health status. Many of them care for HIV infected patients who are in their 60’s and are robust, have had an excellent response to HAART, and are leading active and productive lives. At the same time, we care for HIV-infected patients in their 50’s with substantial cognitive and/or functional impairment and multiple comorbidities. Additionally, the newly diagnosed patients are basically young people in their 20´s with a good educational level and high relationship with new information technologies. These demographic variables5,7, especially being above or below 50 years of age, are particularly taken into account when starting our stratification model 11.
Clinical and healthcare utilization variables have not been traditionally considered when establishing procedures for PC in HIV-positive patients. However, the number of previous hospitalizations was presented as another key factor in the model. It is known that the highest risk of readmission occurs during the first few days after discharge. It is therefore necessary to conduct interventions during admissions12 and in the early days after hospital discharge, to ensure understanding of and adherence to the treatment itself, and thus avoid readmissions13,14. Several studies, like Hirsch J et al13 and March K. et al14, have shown the usefulness of these strategies. This aspect has been taken into account to incorporate this variable model in the design of possible interventions.
In regard to the issue of multiple comorbidities, this is being considered critically important from the perspective of an individual HIV-positive patient15. Schouten J. et al.16 showed that HIV+ patients had a significantly higher prevalence of age-associated non-transmissible comorbidities than uninfected control patients of similar age, in terms of composite comorbidity burden, and more specifically regarding hypertension, cardiovascular and peripheral vascular disease, and impaired renal function.
As we have mentioned throughout this document, HCV coinfection is a key factor in monitoring HIV+ patients, for different reasons such as the evolution and progression of the disease17. Despite the recent arrival of new drugs to treat this disease, with very high rates of sustained virological response, those drugs are not exempt from interactions and increased complexity of pharmacotherapy of patients. This is why the expert panel considers this as a key variable, and it got the highest score in the model, especially when there is undergoing treatment.
Beyond comorbidities, a fundamental issue in HIV-positive patients is the clinical status and increased vulnerability to stressors associated with falls, hospitalization, mortality and physical disability. All these aspects are properly incorporated in the model18. To consider that aspect, the VACS index score was included, which is significantly associated with patient outcome19,20. Additionally, given the simplicity and familiarity of the data for Hospital Pharmacists, it was also taken into account that the patient does not have good control virological (viral load>1000 Copies/mL) to give high punctuation in the score to indicate that close monitoring is required.
Lastly, the medication-related variables should include monitoring the complete treatment of the patients. In this section of the model you can get the highest weight in patient score, a total of 30 points out of 71 possible points. This is due to the important weight of polypharmacy, interactions, poor adherence and suspected drug-related problems when conducting patient monitoring and possible interventions21,22. Polypharmacy appears therefore as the main challenge in the pharmacotherapeutic approach for HIV+ patients in the next years22,23. As Cantudo R. et al.24 indicated the number of concomitant drugs decreased the adherence to ART. Therefore, this would lead to a clinical deterioration that would result in hospitalization, and it determines the need to act on patients25,26.
As it has been shown, the model includes several PC interventions according to a high/medium or low priority level. However, it is needed to demonstrate what the most valuable strategies are, in order to determine which elements of a treatment plan are most important, or have the highest priority for individual patients, determining those priorities for an adult with HIV27-29. This must be based on the applicability of the evidence, the actual absolute risk reduction achieved in studies, the time needed to act in order to observe the benefit, and the individual’s values and preferences7,30. The patient’s values and preferences are critical regarding several aspects: which outcomes are perceived as the most valuable, which burdens they are willing to endure in order to achieve those outcomes, which are their preferences regarding the potential harms associated with the interventions, and finally, how does the level of uncertainty surrounding the reported benefits of a treatment affect their decision-making process.
Limitations
Limitations to our research include some considerations. For example, factors such as HLA and CYP polymorphisms and psychosocial factors continue being important predictors of disease progression, and are considered important in order to design the model, but sometimes these are difficult to collect, and it has been considered that they may impact other markers such as viral load.
In addition to the factors mentioned in the study, there are others which could also determine the risk of drug-related problems. Some are related to people (and their degree of social support, access to health services or functional status) or healthcare organizations (coordination of care and availability of hospital beds). Although the inclusion of such information could improve the predictive ability of this model, its applicability in the real world would be conditioned by the availability of such data, which are usually not recorded in the computer software and medical records.
It is a priority to develop a prospective, randomized, multicenter study to determine the usefulness of this model versus usual practice in a large cohort setting. Future testing should examine how this model and its interventions can increase the effectiveness and safety of treatments, and the contribution to improved outcomes in health and quality of life.
It is needed to conduct a further evaluation of the validity of the model as a tool to identify patients who may benefit from the proposed interventions, because the content validity of the model has only been assessed in a real cohort but within a small sample research. Those patients may or may not comply with the elements of the model, but this may also help us to identify other concepts that contribute significantly to their ability to manage their medication regimen.
Because new information is emerging rapidly in this fast-evolving field, the expert panel considered carefully the best way to update periodically the information in this model. This project was conceived as an evolving effort which would require the addition of new information in order to improve its contents and the proposed interventions.
In conclusion, this study supported the design and adaptation of a selection and stratification model for PC in HIV-positive patients, as a tool to identify those who may benefit from the intervention by Hospital Pharmacists (e.g., risk of drug-related events, to improve adherence).
We believe that this model supports the expansion of Clinical Pharmacist involvement in HIV-positive care centers, in order to establish a selection and stratification model in the interdisciplinary team as the standard for achieving best practice.