Contribution to scientific literature
This work provides information about the prescription of antimicrobials in the real clinical practice in Spain, beyond the recommendations included in the clinical guidelines that, in general, are based solely on the management of the patient colonized by Pseudomonas aeruginosa.
Faculty staff treating cystic fibrosis patients can use this work to know alternative treatment regimens used in Spain for the management not only of P. aeruginosa colonized patients but also of those colonized by other important pathogens such as Burkholderia cepacia or methicillin-resistant Staphylococcus aureus, for which there is no published expert recommendation.
Introduction
Antibiotic treatment against chronic bronchopulmonary infection in cystic fibrosis (CF) patients has substantially contributed to a rise in their life expectancy over the last years, as well as to an improvement in their quality of life1,2. Inhaled antibiotics, in particular, have become the treatment cornerstone of CF patients chronically colonized by Pseudomonas aeruginosa due to their contribution to patients’ lung function preservation and to the reduction in the number of exacerbations3-5.
Antibiotic management in CF patients is, however, very complex. Antimicrobial agents do not often achieve effective concentrations in the lungs due to a high er clearance in these patients. Moreover, they may be ineffective due to the special environment found in the CF lung and to the biofilm-forming ability of CF pathogens; potentially allowing the development of antibiotic resistance3. Inhaled agents partially address these problems, but patients can become refractory to them with continuous treatment or lose their benefits during the resting periods of the on-off cycles6-8. Finally, the emerging role of new CF pathogens, such as Burkholderia cepacia complex (BCC), non-tuberculous mycobacteria (NTM) or methicillin-resistant Staphylococcus aureus (MRSA), in disease progression has made necessary for physicians to test new treatment strategies against them2,9,10.
Thus, antibiotic prescription by CF specialists in “real life” does not always follow the recommendations within consensus guidelines or in drug datasheets. A recent report based on the Cystic Fibrosis Foundation (CFF) Patient Registry data found that CF specialists often prescribe two different inhaled antibiotics in rotational cycles of 28 days to patients chronically colonized by P. aeruginosa11. Also, many case reports describe the use of intravenous-specific antibiotics by the inhaled route against CF pathogens other than P. aeruginosa (or against P. aeruginosa strains refractory to conventional therapies), in an attempt to obtain the same benefits that this type of therapy achieves against the bacteria12.
In Spain there is no national patient registry, although some information about inhaled antibiotic prescription in our country has been published in the European Cystic Fibrosis Society (ECFS) patient registry13. However, the amount of information is scarce and nothing has been reported about treatment regimens, type of antimicrobial agents, etc. Between March to November 2013, we performed a national multicenter study involving the most important CF-specialized units which represented all the different Autonomous Communities in Spain14. Our objectives were not only to determine the Spanish CF pathogens epidemiology, but also to describe the clinical and demographical characteristics of our CF population in the context of an absence of a national registry. In this work, we present the prevalence of the different antibiotic and non-antibiotic therapies administered to these patients in the year prior to their inclusion in the study (2013), with a special focus on the type and mode of administration of inhaled antibiotics.
Methods
The methodology of this prospective, multicenter, observational study has already been published14. Briefly, 24 specialized CF Units for adult (n=12) and pediatric (n=12) patients in 17 Spanish tertiary-care hospitals participated, providing sputum samples and clinical and demographical data from 15 non-selected consecutive patients during their routine follow-up visits. Patients’ data collection included antibiotic therapies prescribed during the year before the recruitment, as well as the use of azithromycin as anti-inflammatory drug and other non-antibiotic therapies like aerosolized hypertonic saline (HS) or dornase alpha (Dα). Antibiotic prescription was also stratified by age (<18 and ≥18 years old, respectively), by pulmonary function measured by the last FEV1 before inclusion (mild disease: ≥70% and moderate-severe disease: <70%) and by P. aeruginosa colonization status (chronic, intermittent or absent) following the modified Leeds criteria15. Proportional Venn and linear diagrams were generated by on-line applets (http://www.eulerdiagrams.org/eulerAPE; https://www.cs.kent.ac.uk/people/staff/pjr/linear/)16,17. The study was approved by each participant hospital’s ethical committee. Written informed consent was provided by all patients and/or by their parents or legal guardians.
Results
Three hundred and forty-one patients were recruited in the multicenter study and their clinical and demographical characteristics have already been published but they are also summarized in Table 1 14. P. aeruginosa colonization status was defined as chronic, intermittent or absent in 158 (46%), 74 (22%) and 109 (32%) patients, respectively. The corresponding numbers for patients <18 years (n=161) were 46 (29%), 41 (25%) and 74 (46%), and for patients ≥18 years (n=180) were 112 (63%), 33 (18%) and 35 (19%), respectively. Nearly all the patients (n=338, 99%) received some type of antibiotic during the year prior to their inclusion in the study (Table 2) and the prevalence of oral, inhaled and intravenous therapies was 89% (n=302), 80% (n=273) and 31% (n=105), respectively. The proportion of patients in whom oral, inhaled and intravenous administration routes were combined is shown in Figure 1. The corresponding diagrams for patients stratified by age, pulmonary function and P. aeruginosa colonization status are shown in Figure 2 Figure 3 and Figure 4.
Table 1 Clinical and demographical characteristics of the patients
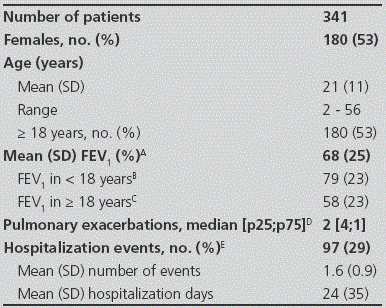
Data available for: A: 330 patients; B: 150 patients; C: 180 patients; D: 312 patients; E: 337 patients.
Table 2 Antibiotic therapies administered to the patients in a one-year period
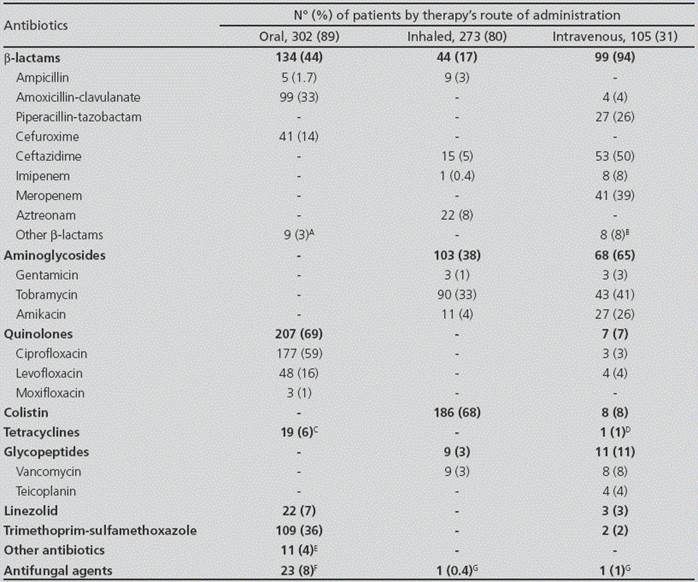
A: Cefditoren (n=3), cefadroxil (n=1), cefaclor (n=2); cloxacillin (n=3); B: Cefoxitin (n=2), cloxacillin (n=2); cefepime (n=4); C: Doxycycline (n=4), minocycline (n=15); D: Tigecycline (n=1); E: Rifampicin (n=6); fusidic acid (n=2), metronidazole (n=1), fosfomycin (n=2), clindamycin (n=1); F: Itraconazole (n=14), voriconazole (n=9), fluconazole (n=1); G: Amphotericin B (n=1). Some patients received more than one oral, intravenous or inhaled antibiotic, which explains why the percentages of each group of antimicrobials (e.g. β-lactams) are not equal to the addition of the frequencies of each individual agent.
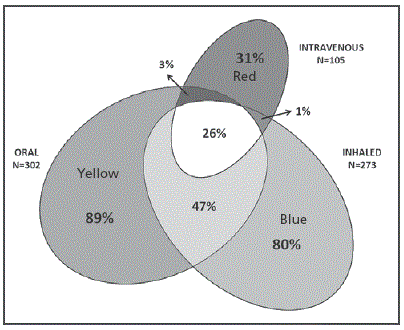
Figure 1 Area proportional Venn diagram showing the annual prevalence of prescribed antibiotics by their administration route. The 3 ellipses correspond to the prevalence of oral (yellow ), inhaled (blue) and intravenous (red) therapies administered to our patients in a one-year period. Overlapping areas identify patients receiving antibiotics by more than 1 administration route.
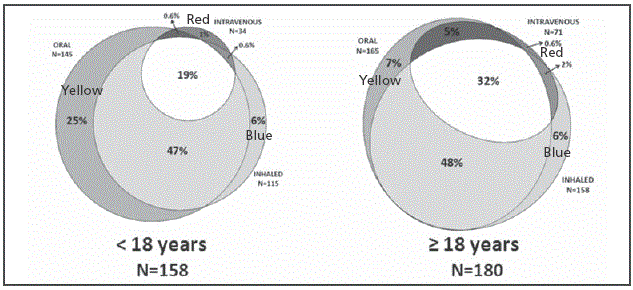
Figure 2 Area proportional Venn diagram showing the annual prevalence of prescribed antibiotics by its administration route among patients stratified by their age. The 3 ellipses correspond to the prevalence of oral (yellow), inhaled (blue) and intravenous (red) therapies administered to our patients in a one-year period. Overlapping areas identify patients receiving antibiotics by more than 1 administration route.
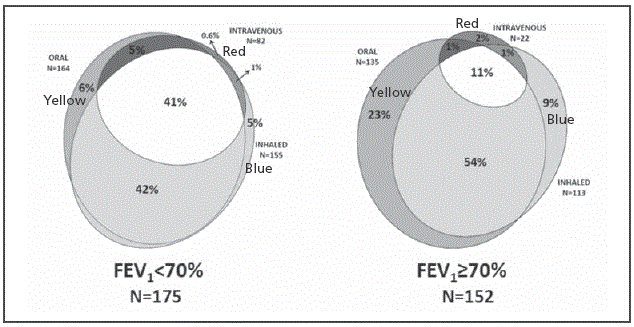
Figure 3 Area proportional Venn diagram showing the annual prevalence of prescribed antibiotics by its administration route among patients stratified by their pulmonary function (FEV 1≥70%=mild disease; FEV1< 70%=moderate-severe disease). The 3 ellipses correspond to the prevalence of oral (yellow), inhaled (blue) and intravenous (red) therapies administered to our patients in a one-year period. Overlapping areas identify patients receiving antibiotics by more than 1 administration route.

Figure 4 Area proportional Venn diagram showing the annual prevalence of prescribed antibiotics by its administration route among patients stratified by their P. aeruginosa colonization status. The 3 ellipses correspond to the prevalence of oral (yellow), inhaled (blue) and intravenous (red) therapies administered to our patients in a one-year period. Overlapping areas identify patients receiving antibiotics by more than 1 administration route.
The most frequent oral agents administered to the patients included ciprofloxacin (59%), trimethoprim-sulfamethoxazole (36%) and amoxicillin-clavulanate (33%). The most prevalent intravenous treatments were ceftazidime (50%), tobramycin (41%), meropenem (39%) and piperacillin-tazobactam (26%) (Table 2). Inhaled and intravenous antibiotics were significantly more often prescribed to patients aged ≥18 years (p<0.001), with P. aeruginosa chronic colonization (p<0.001) and with moderate-advanced disease (p<0.001), and also oral antibiotics were significantly more often prescribed in the last two groups (p=0.006 and p=0.04, respectively).
As to the inhaled antibiotic therapies, the majority of the patients (n=206, 76%) received only one inhaled antibiotic, whereas two or three different inhaled antibiotics were administered in 61 (22%) and 6 (2%) patients, respectively (Figure 5). Among these 67 patients in whom more than one inhaled antibiotic was prescribed, 51(19%) received two antimicrobials concomitantly in a rotating scheme, whereas 5 did not alternate the treatments and no information was available for 11 patients. The prescription prevalence of the three approved inhaled antibiotics in Spain (sodium colistimethate, tobramycin and aztreonam lysine) is shown in Figure 6. Although liposomal amikacin was not approved in our country at the time of the study, it was received by 8 patients in monotherapy as part of a clinical trial. Interestingly, there was a high percentage of patients (14%, n=39) who received intravenous-formulated antibiotics by inhalation, either alone or combined with specific inhaled formulations, the most frequent of them being ceftazidime (n=15, 5%), ampicillin (n=9, 3%) and vancomycin (n=9, 3%). The administration of intravenous-specific antibiotic formulations by the inhaled route and the pathogens associated with these treatment schemes are summarized in Table 3.

Figure 5 Length proportional linear diagram showing the annual prevalence of all the antibiotics prescribed to CF-patients by an inhaled route. Each antibiotic is represented by a horizontal bar. Areas in which lines overlap (depicted in white and pink) represent patients receiving more than one inhaled antibiotic. Numbers up and down the figure represent patients that received one or more inhaled treatments in the year 2013, being in red those taking only one antibiotic, in black those taking two antibiotics and in blue those taking 3 antibiotics. AMK: amikacin; AMP: ampicillin; AZLI: aztreonamlysine; CAZ: ceftazidime; COL: colistin; GEN: gentamicin; TOB: tobramycin; VAN: vancomycin.

Figure 6 Area proportional Venn diagram showing the annual prescription prevalence of the currently approved inhaled antibiotics in Spain. Overlapping areas identify patients receiving more than one class of inhaled antibiotic in the year prior to their inclusion in the study. A: Some patients in this group also received inhaled amikacin (n=2), ampicillin (n=3), ceftazidime (n=1), vancomycin (n=5) and gentamicin plus ceftazidime (n=1). B: A patient in this group also received inhaled ampicillin. C: Some patients in this group also received inhaled ampicillin (n=1) and cezftazidime (n=1). D: Some patients in this group also received inhaled amikacin (n=1) and vancomycin (n=2).
Table 3 Number of patients treated with inhaled intravenous-specific formulations and their targeted CF pathogens
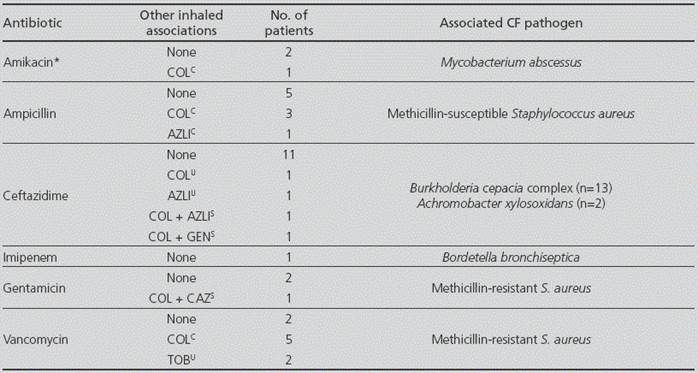
AZLI: Aztreonam lysine; CAZ: ceftazidime; COL: Sodium colistimethate; GEN: Gentamicin; TOB: Tobramycin. C: Concomitant administration. S: Sequential administration. U: Unknown administration. *Refers only to the intravenous formulation of the antibiotic.
Anti-inflammatory azithromycin administered orally to 198 (58%) patients in the year prior to their recruitment was significantly higher among those aged ≥18 years (p<0.001), with P. aeruginosa chronic colonization (p<0.001) and with moderate-advanced disease (p<0.001). The prevalence of other non-antibiotic therapies is shown on Table 4. No significant differences were seen in HS administration between patient groups, whereas Dα administration was significantly higher in patients with moderate-severe disease vs. patients with mild disease (p=0.004). Bronchodilatators (BD) and inhaled glucocorticoids (IGC) were significantly given more to patients aged ≥18 years (p<0.001) and to patients with moderate-advanced disease (p<0.001) and no differences in their use were seen when considering the P. aeruginosa colonization status of the patients.
Discussion
This study provides the first wide report about antibiotic use in the Spanish CF setting in the absence of a national patient registry. It should be stressed that our study population is older and has a more advanced dis ease than that reflected in the ECFS registry, with a median (IQR) age of 18 (28-11) years vs. 15 (26-7.6) years and a median (IQR) FEV1 (%) of 82.5 (92.3-66) vs. 92.5 (104.7-79.2) in <18 years and of 55.6 (73-40) vs. 67.3 (82-48) in ≥18 years (see Figure 7 and Figure 8). The rate of chronic P. aeruginosa colonization is also higher among our patients (46% vs. 28.6%)13. These differences might explain the higher use of Dα and inhaled therapy when compared with the ECFS report (31% vs. 18% and 80% vs. 53%, respectively), as Dα therapy has been traditionally reserved for patients with more advanced disease and inhaled antibiotics are mostly used for the maintenance treatment of chronically colonized P. aeruginosa patients3,4.
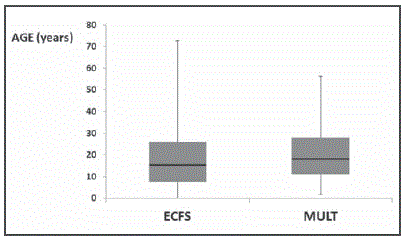
Figure 7 Boxplot analysis of the age of the patients included in the European patient registry (ECFS) and in our study (MULT). Median values are represented as black lines. Percentiles 75 and 25 are the top and the bottom of the colored boxes.

Figure 8 Boxplot analysis of the pulmonary function (FEV1) of the patients included in the European patient registry (ECFS) and in our study (MULT) stratified by their age. Median values are represented as black lines. Percentiles 75 and 25 are the top and the bottom of the colored boxes.
In general, the prescription rates of the different antibiotic therapies registered in this study adjust well with CF treatment guidelines, which are mainly focused in P. aeruginosa eradication, exacerbations and in the lung health maintenance of chronically colonized CF patients3,4,18,19. Not surprisingly, ciprofloxacin is the most common oral drug administered, as this antibiotic is used to treat mild P. aeruginosa exacerbations and can be included in treatment regimens along with inhaled antibiotics for the eradication of this bacteria3,4,18,19. Levofloxacin, which has demonstrated antipseudomonal activity, is also largely used in our country.
Intravenous antibiotic regimens were also common in our patients in parallel with their high rates of chronic P. aeruginosa colonization. In fact, the most common intravenous agents are antipseudomonal β-lactams (meropenem, ceftazidime and piperacillin-tazobactam) and tobramycin, which are normally administered in combination for the treatment of severe P. aeruginosa exacerbations3,19. Sodium colistimethate, which is administered continuously twice a day, and tobramycin and aztreonam lysine, which are administered twice and thrice a day respectively in alternating on/off cycles of 28 days, are currently the only approved inhaled medications for maintenance and eradication therapies in chronically-colonized P. aeruginosa CF patients3. The majority of our patients received only one of these antibiotics in a one-year period, thus, following the indications for P. aeruginosa maintenance treatment3,4. However, one of the most interesting results of this work is the relatively common use of more than one inhaled antibiotic given in a rotational scheme of 28 days. This maintenance strategy, which has been recently introduced in the Spanish consensus guidelines for patients with poor response to standard schemes, has recently been proved to be safe and may provide an additional clinical benefit to CF patients when compared with the recommended intermittent use of inhaled therapy3,20. Continuous rotational treatment may also prolong the lifespan of inhaled therapies by preventing the emergence of antibiotic resistance among P. aeruginosa strains8. This complexity in the treatment strategies for chronically colonized P. aeruginosa patients is also reflected in Figure 4, in which it is evident that combination strategies are more common in patients colonized by P. aeruginosa.
Another important finding of the study is the relatively high number of patients treated with intravenous formulated antibiotics using the inhaled route. This finding reflects clinicians’ necessity of treating pathogens other than P. aeruginosa in CF patients, either as a maintenance therapy or as part of eradication or exacerbation regimes. The problem is that, even when virtually every antibiotic could be used by this route, non-specific inhaled preparations administered by non-adequate devices and conditions could lead to a worse toleration of the therapy and to the achievement of fewer antibiotic concentrations in the lung21,22. Although some new inhaled formulations of levofloxacin and vancomycin are now being tested in CF-patients23,24, more clinical trials are needed to assess the safety and efficacy of new inhaled antibiotics against CF pathogens as MRSA, BCC or NTM. These data would permit the inclusion of new recommendations against these pathogens in CF treatment guidelines.
Even though the prevalence of MRSA is not so high in our country (around 11%)14, it is an important CF pathogen associated with increased morbility and mortality among colonized CF-patients25. The most frequently prescribed oral agents among 46 patients in our study with any record of MRSA isolation in 2013 were trimethoprim-sulfamethoxazole (n=20) and linezolid (n=17), and the treatment periods allowed us to infer that they were used to treat MRSA exacerbations. Although there are no consensus treatment guidelines for MRSA exacerbations, this is in line with general recommendations that identified linezolid as a preferred first-line treatment option over intravenous vancomycin or teicoplanin due to its lack of nephrotoxic effects, especially for patients treated with aminoglycosides2. In fact, few patients received intravenous vancomycin (n=4) or teicoplanin (n=3), which were probably used to treat severe exacerbations. The wide use of trimethoprim-sulfamethoxazole to treat mild MRSA exacerbations is explained by its oral bioavailability, its favorable lung penetration and activity against MRSA and other CF pathogens as BCC or Stenotrophomonas maltophilia. However, it could also select S. aureus Small Colony Variants (SCVs) which are associated with worse course of the disease2,26. There are no consensus guidelines referring to continuous maintenance treatment against MRSA. However, we identified, 5 patients receiving continuous inhaled vancomycin which reflects, as mentioned above, the effort of clinicians to improve the conditions of their patients using any available option. Four patients received inhaled vancomycin in short cycles combined with oral regimens containing rifampicin with fusidic acid and/or trimethoprim-sulfamethoxazole or linezolid. This was identified as MRSA eradication attempts as recommended in the UK CF guidelines and in other clinical reports25,27,28.
On the other hand, BCC infections are also of concern for CF-patients due to their negative impact in lung function deterioration, spreading potential and high intrinsic resistance to many of the available antibiotics29. However and probably due to their low prevalence in CF patients, BCC-specific recommendations about any treatment aspects (maintenance, exacerbation or eradication) are lacking in clinical guidelines, forcing clinicians to assess the treatment of each patient individually10. Treatment options used by clinicians to treat this pathogen include multiple combinations of oral (trimethoprim-sulfamethoxazole, minocycline), intravenous (meropenem, ceftazidime) or inhaled (meropenem, tobramycin) therapies10. In our study, the prescription of oral and intravenous agents to patients with any history of BCC isolation (n=40) adjusted to this trend but, surprisingly, many of them received courses of inhaled ceftazidime (n=12) rather than meropenem (n=0) and at least 3 patients were taking this drug continuously as a maintenance treatment. There is limited literature about the use of inhaled ceftazidime in CF patients and there is only one case-report in which inhaled ceftazidime was successfully used for a post-transplant eradication of a B. cenocepacia strain12. The efficacy of inhaled ceftazidime containing regimens should be evaluated in patients carrying BCC species due to the high use of this strategy in our country.
Finally, the treatment of pathogens like Haemophilus influenzae, methicillin-susceptible S. aureus (MSSA) or Streptococcus pneumoniae is reflected in the common use of other oral antibiotics, as amoxicillin-clavulanate, trimethoprim-sulfamethoxazole or cefuroxime5. Use of these antibiotics was more common among younger CF-patients (<18 years) or when no other pathogens were recovered from sputum (data not shown). Maintenance therapies with continuous amoxicillin-clavulanate and trimethoprim-sulfamethoxazole were also identified in 5 and 2 patients respectively, even though some reports relate these antistaphylococcal antibiotics with a higher risk of P. aeruginosa acquisition in CF-patients25. Another interesting finding was the relative common use of inhaled ampicillin in 9 patients chronically colonized by MSSA, 8 of them receiving it continuously. This long-term use of inhaled ampicillin in CF patients has been previously reported and, although it has achieved a reduction in hospitalization rates and oral antibiotic use, it has not been able to eradicate the pathogen12,30.
This work has limitations, principally the lack of information about treatment objectives for each patient in some cases. Recovery of this information was not the main objective of the above mentioned multicenter study and was not included in its design. Therefore and even having information about dosing and duration of each drug, in many cases we were not able to ascertain if a particular treatment was used for maintenance purposes, for the eradication of a particular pathogen or for the management of an exacerbation. Despite this limitation, we obtained relevant information about how antibiotics are used in the Spanish CF context, which could be useful for future comparative studies on this topic in our country and after publication of our national guidelines3. This information also completes that provided by the ECFS patient registry, in which only data about the use of inhaled antibiotics, anti-inflammatory macrolides, Dα, HS and BD are provided14. Future studies on this topic will be needed in Spain to evaluate the treatment role of the new antibiotics, as inhaled levofloxacin and amikacin or intravenous ceftaroline, and the new CFTR modulators, as ivacaftor or its combination with lumacaftor.















