Introduction
Overactive Bladder Syndrome (OBS) is defined as the urgency to urinate, with or without incontinence, along-side an increase in the frequency of micturitions and nocturia, in the absence of urinary infection and other conditions. Its etiology is not completely clear, but it is known that there is an increase in the activity of the detrusor muscle1-2.
The prevalence of OBS is estimated as 11.8% of the overall population, with similar rates in women and men; it affects >400 million persons in the world 1. Its prevalence increases with age, and there are 30-40% of >75-year-old people affected1.
Even though this disease is not life-threatening, it represents a problem with a major negative impact on the quality of life of patients and their relatives or carers, because it affects social and sexual functions, personal relationships, and everyday working life1-2.
The initial treatment for all patients with urinary incontinence includes lifestyle changes and behavioural therapy (bladder training, pelvic floor muscle exercises, etc.)1-2-4. Behavioural therapies can be used in combination with drug treatments4.
Antimuscarinic drugs represent the cornerstone of drug treatment for OBS1-3-4. They act by blocking the muscarinic receptors in the bladder wall, reducing the contractibility of the detrusor muscle. Mouth dryness is their main adverse effect, though they can also cause constipation, blurry vision, fatigue, and cognitive dysfunction.
Mirabegron is the first in a new class of drugs in the group of selective β3-adrenoceptor agonists, which prevail in the detrusor muscle of the bladder. Their bladder activation facilitates urine storage, because it causes the relaxation of the smooth muscle of the bladder. It is considered that this allows to increase the bladder capacity and to reduce the frequency of contractions and, therefore, of involuntary micturitions2.
Objective
The objective of this study is to review the scientific evidence available on mirabegron, with the aim to analyze its efficacy, safety and cost, and thus estimate its role within current pharmacotherapy.
Materials and methods
The efficacy and safety of mirabegron were analyzed through an assessment of scientific evidence using browsers of primary bibliographic sources. On June, 2015, a search was conducted in the Trip Data Base and Pub-Med medical databases, using the term “mirabegron”. In total, 933,208 articles were identified. The following search filters were subsequently applied in order to select the most adequate articles:
On one hand, the following filter was applied: Clinical Trial, full text, publication date within the last five years, and humans: 34 results were obtained.
On the other hand, they were filtered by Review, full text, publication date within the last five years, and species in human: 39 results were identified.
There was also a review of the different clinical guidelines1-2-3-4-10-12 available on the treatment of Overactive Bladder Syndrome, as well as the product specifications for mirabegron 5.
Additionally, the Report by the EPAR (European Public Assessment Reports) about this drug was analyzed2, in order to select all those clinical trials that were used by the European Medicines Agency for the approval of its marketing authorization.
The cost of the different pharmacological alternatives was assessed according to their Defined Daily Dose (DDD), obtained from the WHO Collaborating Centre for Drug Statistics Methodology, reviewed on June, 2015. The cost obtained from the BotPlus program was considered; this was reviewed on June, 2015. For those molecules with different formulations, such as the case of generics, the cost of the cheapest formulation was considered.
Results
Three randomized clinical trials (RCTs) (Phase III), placebo-controlled, double-blind, and with 12 weeks of duration, support the use of mirabegron for the treatment of OBS (ECA CL-046, CL-047 and CL-074)6-7-8. Their characteristics appear in Table 1.
Before each study, there was a preinclusion double-blind period of 2 weeks in order to select patients; after this, patients were randomized to the drug under study.
The patients selected were ≥18-year-old men and women, with OBS symptoms for at least 3 months, with a mean frequency of ≥ 8 micturitions/24 hours, and at least 3 urgency episodes (grades 3 or 4), with or without incontinence, collected in a 3-day urination diary during the preinclusion period. Those patients with stress or mixed incontinence with predominant stress were excluded from the study, as well as those with a mean> 3.000 ml volume of urine per day. Patients with severe hypertension and clinical ECG alterations were also excluded.
The number of patients included in the three clinical trials was 4,622. Patients were randomized to receive mirabegron 25, 50 or 100mg vs. placebo. In the CL-0468 study, Tolterodine 4mg was also included as active control, but there was no statistical comparison vs. mirabegron, because the clinical trial was not designed for this. The majority of the patients included in the studies were women (72-83%), with a mean age of 59-to-61-years. They presented a mean frequency of 11-12 urinations per day, with 2-3 incontinence episodes (in the incontinent sub-group, which represented 59% in two of the clinical trials, and 70% in the other). Around 49-60% of patients had been previously treated with anticholinergic agents.
The design of pivotal studies, including objectives, inclusion and exclusion criteria, primary and secondary efficacy variables, is acceptable, and in general coincide with the Guidelines by The Committee for Medicinal Products for Human Use (CHMP). The population also seems to be adequate and representative.
Efficacy
All studies share the same primary efficacy variables6-7-8:
Changes from baseline to the final visit in the mean number of incontinence episodes within 24 hours, based on the entries in the urination diary 3 days before each control visit. Only in the sub-group of incontinent patients.
Changes from baseline to the final visit in the number of urinations at 24 hours, based on the entries in the urination diary 3 days before the control visit.
The secondary variables included 6-7-8: changes from baseline to the final visit in the mean volume per urination, change in the mean number of urinations per 24 hours at 4 weeks, change in the mean number of incontinence episodes per 24 hours at 4 weeks, and percentage of responders with no episodes.
Quality of life was assessed by using OBS questionnaires assigning scores to aspects such as concern about the symptoms (range 0 to 100), Patient Perception of Bladder Condition (PPBC) and Visual Scale of Satisfaction with Treatment (TS-VAS, range 0 to 10)6-7-8.
Results of the studies:
Number of incontinence episodes per 24 hours: The mean number of incontinence episodes within 24 hours at baseline was comparable in all studies. All mirabegron groups showed a reduction in the number of incontinence episodes per 24 hours in the final visit vs. placebo.
The mean reduction in the number of episodes was -1.10, -1.49 and -1.50 for placebo, mirabegron 50mg and mirabegron 100mg, respectively. The differences in the reduction vs. placebo were -0.40 (mirabegron 50mg) and -0.41 (mirabegron 100 mg)2. These differences, though statistically significant, had little clinical relevance. The difference between tolterodine and placebo was not statistically significant (-0.10)2.
Number of urinations per 24 hours: The mean number of urinations within 24 hours at baseline was comparable in all studies. All mirabegron groups showed a reduction in the number of urinations per 24 hours vs. placebo.
The mean change was of -1.20, -1.75 and -1.74 for placebo, mirabegron 50mg and mirabegron 100mg, respectively. The differences in reduction vs. placebo were -0.55 (mirabegron 50mg) and -0.54 (mirabegron 100mg)2. These differences, though statistically significant, had little clinical relevance. The differences between tolterodine and placebo (0.25) were not statistically significant.
Volume passed per urination in 24 hours: The mean volume per urination at baseline was comparable in all studies. All mirabegron groups demonstrated an increase in the volume passed per urination vs. placebo.
The mean change was of 9.4, 21.4 and 21.7 mL for placebo, mirabegron 50mg and 100mg, respectively. The mean difference compared vs. placebo was 11.9 mL (mirabegron 50mg) and 12.3 mL (mirabegron 100mg)2. These differences were statistically significant both with mirabegron and tolterodine.
There were no significant differences in terms of the number of responders with no incontinence episodes at 12 weeks between mirabegron and placebo, or between tolterodine and placebo2.
No dose-dependent effect was observed, because the 100mg dose did not demonstrate being better than the 50mg dose. However, the 25mg dose did show lower effect vs. the 50mg dose2.
Regarding patient perception about the improvement in their quality of life, mirabegron demonstrated a reduction in the Scale of Concern about Symptoms (improvement) compared with placebo, as well as in Patient Perception of Bladder Condition2.
Long-term efficacy data are based on a 12-month study on safety (CL-049)9 which included 2,452 patients (part of these came from previous studies), where efficacy outcomes were measured as secondary variables, and the highest evidence was obtained in the non-formal comparison between mirabegron and tolterodine. The reduction in the number of incontinence episodes per 24 hours was -1.01 with mirabegron 50mg and -1.26 with tolterodine2. The change in the number of urina-tions per 24 hours was -1.27 with mirabegron 50mg and -1.39 with tolterodine. Even though the results obtained don’t suggest an efficacy loss, the lack of a placebo arm and of statistical comparison does not allow to reach any conclusions about the sustained effect of mirabegron2. Even so, the comparison of effects between mirabegron and tolterodine reveals a similar effect both at short term and long term.
Summing up, an effect in favour of mirabegron can be observed when analyzing all studies, both for primary and secondary variables, which is statistically significant in the majority of cases. The comparison with tolterodine shows a similar effect. The effect of mirabegron is significant but modest, and in line with the rest of medications approved for this indication2-14-16.
Safety
The safety data available are based mostly on the exposure of patients who participated in the 12-week pivotal clinical trials 6-7-8, and on the patients included in the long-term 12-month study, where the primary variable was safety9.
In the three placebo-controlled studies at 12 weeks2-6-7-8, 88% of patients completed treatment with mirabegron, and 4% abandoned the study due to adverse events. The majority of adverse reactions were mild to moderate. During this short-term exposure, 53.4% of patients reported some adverse effect (55.2% with tolterodine and 60.2% with placebo). The most frequent adverse effects were: nasopharyngitis (7.4%), hypertension (5.2%), increase of glucose in blood (5.7%), headache (3.1%), urinary tract infections (2.9%), constipation (2.1%) and tachycardia (1.2%). Tachycardia led to treatment discontinuation in 0.1% of cases. Severe adverse reactions included atrial fibrillation (0.2%). Mouth dryness was much more frequent in the tolterodine 4mg arm (mirabegron 1.7% vs. tolterodine 10.4%).
When assessing long-term safety9, the percentage of patients with 1 or more adverse effects was 60.5% for mirabegron and 62.6% for tolterodine. The most frequent adverse reactions were similar between groups, except for mouth dryness: 8.6% with tolterodine vs. 2.8% with mirabegron 50mg. Severe adverse events were reported in 5.2% and 6.2% of patients on mirabegron (50 and 100mg), and 5.4% of patients with tolterodine. Treatment discontinuation as a consequence of adverse reactions occurred in 6.4% and 5.9% of patients on mirabegron (50 and 100 mg) and in 6.0% of patients on tolterodine.
The incidence of adverse effects leading to treatment discontinuation was similar in all groups (including placebo). There were no qualitative differences between exposures at short vs. long term2.
Cardiovascular events were closely watched during the studies. Mirabegron showed a modest increase in pulse and arterial blood pressure (1 bpm and ≤ 1 mm Hg vs. placebo); these were reversible after treatment interruption2. Regarding the effect on the QT interval, the 50mg dose seems to be safe, and has not shown a clinically relevant QT interval prolongation. However, the clinical trials did not include patients with previous QT interval prolongation or patients on treatment with medications that lead to QT interval prolongation; therefore, the effect on this type of patients is unknown, and caution is recommended2. Moreover, it has not been evaluated in patients with severe non-controlled hypertension (SBP ≥ 189 mm Hg and/or DBP ≥ 110 mm Hg); therefore, it is not recommended to use it in this type of patients. There are limited data for patients with Stage 2 hypertension (SBP ≥ 160 mm Hg or DBP ≥ 100 mm Hg).
In total, there were 34 cases of hypersensitivity (23 in short-term studies and 11 during the long-term studies). The incidence and severity of these reactions was 2-3 times higher with the 100mg dose of mirabegron than with tolterodine or placebo.
The Monthly Newsletter of February, 201617 by the Spanish Agency of Medicinal Products and Medical Devices (AEMPS) includes any new safety information derived of the evaluation od periodical safety reports. Regarding mirabegron, there were reports of hypertension episodes, constipation, diarrhoea, headache and dizziness.
As a result of the severe cases of hypertension and blood pressure increase in patients under treatment with mirabegron, the AEMPS sent a letter about safety to healthcare professionals 18, reminding them that its use is contraindicated in patients with severe uncontrolled hypertension, and recommending to measure blood pressure before initiating treatment, and to conduct regular controls, particularly in patients with hypertension.
Special precautions for use:
Renal impairment: Mirabegron has not been studied in patients with end-stage renal disease (GFR < 15 ml/min/1.73 m2 or haemodialysis) and, therefore, its use is not recommended in this population. There are limited data for patients with severe renal impairment (GFR from 15 to 29 ml/min/1.73 m2). For this population, it is recommended to reduce the dose to 25 mg2-5 (this strength has not been marketed, and 50mg tablets cannot be split). Its use is not recommended for patients with severe renal impairment (GFR from 15 to 29 ml/min/1.73 m2) who are receiving concomitant treatment with potent CYP3A inhibitors.
Liver impairment: Mirabegron has not been studied in patients with severe liver impairment (Class C Child-Pugh) , and therefore its use is not recommended in this patient population. Its use is not recommended either for patients with moderate liver impairment (Class B Child-Pugh), who are under concomitant treatment with potent CYP3A inhibitors. The recommended dose for patients with moderate liver impairment (Class B Child-Pugh) is 25 mg2-5.
Fertility, pregnancy and breastfeeding: The effect of mirabegron on human fertility has not been determined. There are limited data about its use in pregnant women. Its use is not recommended during pregnancy, or for women of childbearing age who are not using contraceptive methods2-5. No studies have been conducted to evaluate the impact of mirabegron on milk production in humans, its presence in human milk or its effects on the breastfed baby; therefore, it should not be administered during the breastfeeding period 2-5.
Interactions:
No clinically relevant pharmacological interactions are expected between mirabegron and medications that are inhibitors, inducers and substrates for CYP450 isoenzymes or transporters, except in the case of CYP2D6 substrates2-(5.
Mirabegron has moderate inhibitor potency on CYP2D6. Caution is recommended, if administered concomitantly with medications with narrow therapeutic range and that are significantly metabolized by CYP2D6, such as thioridazine, Type 1C antiarrhythmics (for example, flecainide, propafenone) and tricyclic antidepressants (for example, imipramine, desipramine) 2-5.
Effect of mirabegron on transporters: It is a weak P-gp inhibitor. For patients initiating treatment with a combination of mirabegron and digoxin, the lowest dose of digoxin should be initially prescribed, and then its serum concentrations should be monitored for dose adjustment. The potential of mirabegron for P-gp inhibition should be taken into account when used in combination with P-gp sensitive substrates, such as dabigatran2-5.
RISK MANAGEMENT PLAN BY THE EUROPEAN MEDICINES AGENCY
This medication is subject to additional follow-up, in order to detect new safety information. All healthcare professionals are invited to report any suspected adverse reactions 2-5.
The risk management plan by the EMA includes two identified major risks (increased heart rate and tachycardia, and hypersensitivity reactions) and five potential major risks (QT prolongation, hypertension, urinary tract infections, embryo-fetal toxicity, and concomitant treatment with CYP2D6 substrates with narrow therapeutic range). They consider that there is lack of information for some situations (end-stage renal disease, cardiovascular and a higher risk to develop heart failure, in Paediatrics and lymphocyte reduction). In all these cases, they recommend routine pharmacovigilance, and they also recommend conducting a post-approval study in order to research cardiovascular safety, particularly in elderly patients 2-5.
Discussion
Mirabegron represents a new option for the treatment of Overactive Bladder Syndrome, with a new mechanism of action (β 3-adrenoceptor agonist)2-14.
It has demonstrated a beneficial effect on the main symptoms of OBS, such as the reduction in frequency of urinations, in the number of incontinence and urgency episodes, and an increase in the volume of urine per urination. However, this effect is modest and comparable with that achieved by other agents approved for this indication (for example, antimuscarinic drugs).
There are limited long-term efficacy data. The studies conducted with this drug have short duration, which makes it difficult to evaluate the real efficacy of mirabegron at long term. There is a high response to placebo, and therefore the absolute improvement with mirabegron is very small and might not be clinically relevant in everyday clinical practice2. In clinical trials, it has not even reduced one incontinence episode per day vs. placebo; and regarding the number of urinations / day, it has not achieved a reduction of one urination per day over placebo, in patients with a mean of 11-12 urinations per 24 hours.
Besides, no comparative studies have been conducted vs. anticholinergic drugs. Indirect comparisons show that the extent of the effect of mirabegron is similar to that of other drugs used for the treatment of Overactive Bladder. The NICE guidelines14 have positioned it as an alternative option in patients for whom anticholinergic drugs are contraindicated, not clinically effective, or cause unacceptable adverse effects.
In terms of its profile of adverse effects, mirabegron seems to be well tolerated. Overall, it shows a safety profile comparable with the one shown by anticholinergic drugs. It causes less mouth dryness than tolterodine, but there are no differences in the number of treatment discontinuations due to adverse reactions. Unlike anticholinergic drugs, caution is required when administered with medications with narrow therapeutic range that are metabolized by CYP2D6. In terms of cardiovascular safety, it causes a modest increase in pulse and blood pressure. The Risk Management Plan by the EMA describes as major risks the increase in heart rate and tachycardia, and hypersensitivity reactions 2-5.
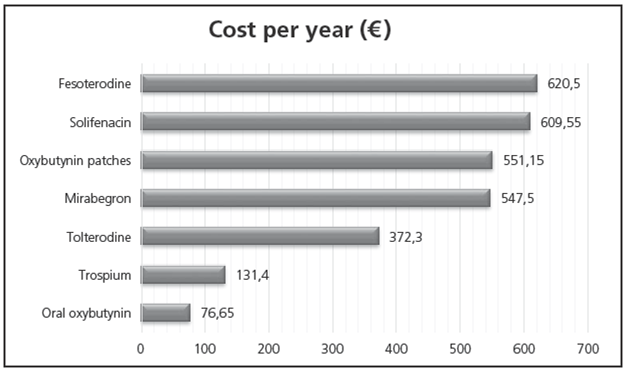
Mirabegron presents a cost similar to that of solifenacin and fesoterodine, but it is more expensive than tolterodine, trospium and oxybutynin.
The conclusion is that mirabegron does not offer any additional benefit in terms of OBS treatment, and therefore does not represent a therapeutic breakthrough. Its clinical efficacy is very modest, and comparable to that achieved with the rest of drugs approved for this indication, and its cost is higher than that for other therapeutic options. Moreover, it will only be possible to consider it as an alternative to anticholinergic drugs when these are contraindicated, lack clinical efficacy, or cause unacceptable adverse effects, due to its cardiac risks, urinary infections, and the uncertainty about its long-term safety.











 texto en
texto en