Introduction
The formal document of information about a medication is the Product Specifications, prepared by the pharmaceutical company that markets it, once evaluated and approved by the Healthcare Authorities. This document represents the basic information, targeted to healthcare professionals, about how to use the medication in an efficacious and safe way. The main support for this healthcare authorization is based on the assessment of data provided by clinical trials for one or more therapeutic indications; therefore, the use of drugs under the condition described in their product specifications has the guarantee of the scientific evaluation for their efficacy and safety. However, these cannot be guaranteed when the drug is prescribed differently from what appears in said document.
The use of medications for indications other than those included in their product specifications is not an infrequent practice, in the outpatient setting1, and most of all in the hospital setting, particularly in areas such as Oncology2 and Psychiatry3. These prescriptions are called “off-label” in international literature, and also include those conducted in population groups different from the ones in the product specifications, particularly in children4,5. There are various reasons that can justify these off-label uses: the existence of therapeutic gaps, the strong research conducted in certain areas and, on the other hand, the lack of interest by some companies about extending their treatment indications, particularly for old medications with low commercial margin.
This practice can offer benefits, but also risks; in fact, there are published studies showing a higher rate of adverse reactions when medications are used off-label6-8. Moreover, the use of medications in non-authorized indications is not always supported by a good level of evidence1,9, and this makes it necessary to conduct a careful assessment of the benefit/risk balance of treatments.
Royal Decree 1015/200910 was the first law enforced about the availability of medications in special situations in Spain; for the first time, three situations were defined: compassionate use of medications under research, the use of medications in situations other than those authorized, and access to medications not authorized in Spain. This regulation clarified the previous situation of overlapping applications, and accelerated its management through planning specific procedures, and determining that the use of an authorized medication in different conditions to those authorized fell within the area of clinical practice; therefore, from the date of enforcement of this Royal Decree, no case-by-case authorization was required from the Spanish Agency of Medications and Medical Devices (AEMPS)11. Before said Royal Decree was published, the procedure for medications under research was similar to that for medications already authorized, and this generated confusion among healthcare professionals. It is important to remember that under this new legislation, the physician responsible must justify any off-label use in the clinical record, respect patient’s autonomy by requesting their informed consent, and take into account any restrictions linked to the prescription and/or dispensing of the medication, as well as reporting any suspected adverse reactions10.
Though in theory off- label uses are exceptional, these are very frequent in many hospitals, and it is also undeniable that new treatment strategies are being developed in certain medical areas much faster than changes occur in medication authorizations; that is why we decided to conduct this study with the objective of describing the extent and profile of off-label uses in a third-level hospital, to analyze the level of scientific evidence supporting said indications, to evaluate the research activity, and to determine the uses finally authorized as new indications at five years after the application.
Methods
A transversal study including all applications for off-label use presented during 2010 at the Hospital Universitario Reina Sofía in Córdoba. The data source was the registry for request of medications received at the Pharmacy Unit for conditions different to those authorized in the product specifications. The Hospital Pharmacy and Therapeutics Committee (PTC) processed and assessed the applications for off-label use. There was a list with those off-label uses previously assessed and approved; therefore, if an application was included in this list, it was authorized automatically. If the application was not included or was new, the requesting physician should provide bibliographic support for the justifying clinical report, and all this was assessed by a Sub-PTC, that sent their evaluation report to Management in order to approve the use or not; and finally, to the following regular PTC meeting for its final evaluation. In case of a favourable report, the indication was included in the positive list. At the beginning of the study period, this list included 208 uses. On the other hand, it was determined that the PTC could request, whenever they considered it relevant, to follow-up the clinical outcomes and/or adverse effects of any off-label use. All this procedure had been informed to the professionals through the hospital intranet, as well as the recommendations included in the RD 1015/2009.
The following data were collected for each application: clinical area, medication, year of authorization, pharmacological group and subgroup according to the ATC Classification (Anatomical Therapeutic Chemical Classification System), off-label indication or indications, level of evidence for each application, on-going clinical trials for the specific indication, and variations in product specifications after five years.
Evaluation of the Level of Evidence. A bibliographic search was conducted in the Pubmed database for each clinical indication. Subsequently, the evidence available was classified according to the criteria published by SIGN (Scottish Intercollegiate Guidelines Network)12, and also used by NICE (National Institute for Health and Care Excellence) for intervention studies. The levels of evidence of the CEBM (Centre for Evidence-based Medicine, Oxford)13 were also used as the other criterion of reference. This evaluation of the evidence available was conducted at two time-points: the year of application (2010) and the year of study completion (2015), with the objective to assess any potential changes or improvements over time. Finally, in order to put into operation and sum up in a simpler way all these levels of evidence, these were grouped secondarily into two categories: good or acceptable level of evidence (at least one clinical trial) and low level of evidence (the rest of designs); these synthesis strategy was also used by other authors1,14-15.
Existence of clinical trials. The International ClinicalTrials.gov Registry was consulted in order to find any on-going clinical trials about the use of the medication for the indication requested16.
Variations in Product Specifications. In order to evaluate if the off-label uses were finally authorized, there has been a review during these 5 follow-up years of the product specifications17 of the medications involved, as well as a periodical review of the Monthly Newsletter by the AEMPS18.
Consistently with the study objectives, there was a descriptive analysis of all the variables collected. The project received a positive decision by the Ethics Committee.
Results
During 2010, there were 190 applications for off-label use of drugs, involving 44 medications and up to 82 different clinical indications.
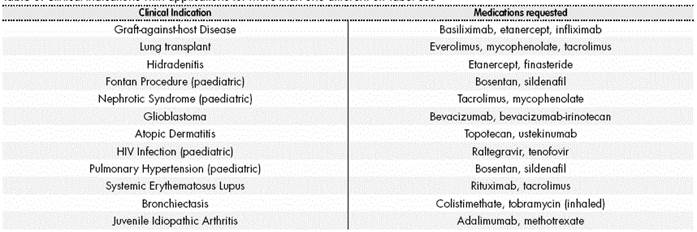
The clinical unit with the highest number of applications was Pulmonology (32%), followed by Paediatrics (22%), Dermatology (10.5%) and Haematology (7.3%). The pharmacological group more requested for off-label use was L (antineoplastic agents and immunomodulators), not only in terms of number of drugs (n=28, 63%), but also and mostly in terms of the number of different indications (n=60, 73.2%) and applications (n=131, 69%). The rest of the ATC groups contributed to a lower number of applications and lower variety of medications (Table 1). The medication with the highest number of individual requests was tacrolimus, followed by mycophenolate, colistimethate and everolimus.
The majority of medications were relatively recent, and had received authorization after the year 2000 in 66% of cases (n=29).
The number of off-label indications for each drug ranged from one single condition (n=27, 61.3% of medications) up to ten different indications for the same medication, such as happened with mycophenolate (Table 2). On the other hand, there were also clinical conditions for which more than one off-label use was required (Table 3); however, this was only one in the majority of cases (64.6%).
Evaluation of the Level of Evidence
At the time of application, over half (n=43, 52.4%) of off-label uses were supported by at least one clinical trial, or what could be considered a good level of evidence (levels 1++, 1+ and 1-, according to the SIGN-NICE scale). However, the level of evidence was low in 47.6% of cases, and a great part of the information available was only about cases and series of cases (Table 4). The most usual situation was for indications supported by low-quality clinical trials (for example, basiliximab in graft-against-host disease, or rituximab in Sjögren’s Syndrome). A very representative example of studies published with very good methodological quality was those for methotrexate in juvenile idiopathic arthritis. And on the other hand, with practically no previous information available, there were applications for nilotinib for acute lymphoid leukemia, and sirolimus for pulmonary arteriovenous fistula, both for paediatric patients; or topotecan and ustekinumab for refractory atopic dermatitis in adult patients.
Table 4 Level of evidence of off-label uses at their time of request (2010) and changes (new levels achieved) after 5 years (2015)
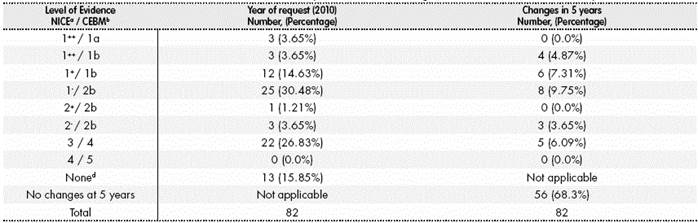
a SIGN-NICE levels of evidence for intervention studies: 1++(high-quality meta-analysis, systematic reviews of randomized clinical trials -RCT-, or RCT with very low risk of bias); 1+ (the same as 1++ with low risk of bias); 1-(the same as 1++ with high risk of bias); 2++(systematic high-quality reviews of studies of cohorts or control-cases, or high-quality cohorts or control-cases, with very low risk of confusion, bias or hazard, and high likelihood of casual relationship); 2+ (studies of cohorts or control-cases well conducted, with low risk of confusion, bias or hazard, and a moderate likelihood of casual relationship; 2- (cohorts or control-cases with high risk of bias); 3 (non-analytical studies; cases and series of cases); 4 (expert opinion). b CEBM, Oxford: 1a (systematic review of RCT, with homogeneity; comparable results and in the same direction); 1b (individual RCT); 1c (efficacy demonstrated by clinical practice and not by experimentation); 2a (systematic review of cohort studies, with homogeneity); 2b (individual study of cohorts and low-quality RCT); 2c (research of health outcomes); 3a (systematic review of control-cases, with homogeneity; 3b (individual control-cases); 4 (series of cases and studies of low-quality cohorts and control-cases); 5 (opinion by experts). c Number of applications that achieve at 5 years the specific level of evidence stated (starting from lower levels in 2010) d No evidence found, including series of cases published in indexed journals.
After assessing the evidence 5 years after the application, two thirds of the off-label uses have not incorporated studies with better quality (n=56, 68.3%). However, 26 of these uses (31.7%) had an improvement in their level of evidence (Table 4); four indications stand out because they acquired level 1++ (such as everolimus for liver transplant, or rituximab for mantle cell lymphoma).
Focusing on the most requested medications, tacrolimus and mycophenolate, very different levels of evidence were found according to the indication for which they were prescribed (Table 5). There was a very good level of evidence for tacrolimus when used for lung transplant, but this was not the case for its use in systemic lupus erythematosus (SLE). In the case of mycophenolate, there was high evidence for its use in lupus nephritis (1++), and very low for interstitial pneumonitis in children.
Table 5 Detailed levels of evidence for each indication of the two medications with the highest number of applications (tacrolimus and mycophenolate)
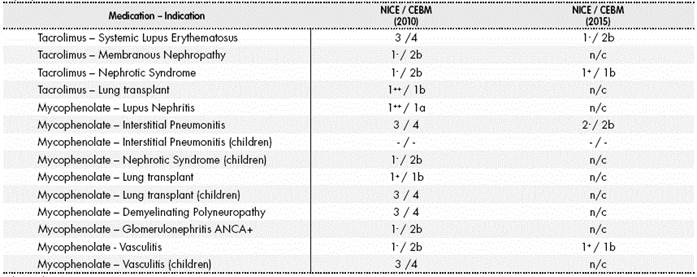
n/c: no changes. (-): no published data available.
Existence of Clinical Trials
For 33% of the indications, no study was found in the International ClinicalTrials.gov Registry; but registered and ongoing clinical trials have been found for the rest of off-label uses. There were usually from 2 to 4 studies, except for some exceptional cases where around 25-30 clinical trials have been detected. Some examples of the latter were the use of bevacizumab for ovarian cancer and glioblastoma, lenalidomide for myelodysplastic syndrome, or rituximab for mantle cell lymphoma.
Variations in Product Specifications
Eleven per cent (n=9 11%) of off-label uses have been authorized as new indications (Table 6), and this extension has been included in the product specifications. These nine indications presented a good level of evidence at the time of application for the requested indication, except for the cases of sildenafil and raltegravir, which reached this level subsequently.
Discussion
It is interesting to conduct research on the use of medications, particularly of some groups such as biologics, and even more when these are used in conditions or indications not appearing in their product specifications, and sometimes in a manner not well supported. On the other hand, through this type of studies we can learn about and get closer to innovative therapeutic approaches, arising from the need to offer some treatment to patients with diseases that have limited alternative options, and often low prevalence. To assess the level of scientific evidence for these uses, contributing with a follow-up of research activity, and to analyze the final inclusion or not of the new indication in the product specifications, represents an innovative approach.
In agreement with other authors, we consider that this type of studies can become a tool for clear identification of multiple and future research initiatives, essentially focused on those off-label uses with limited scientific support19.
Regarding limitations, we think that the outcomes of this study might not be generalizable, mostly because this is a procedure with great variability, not only inherent to medical decisions, but also attributable to the differences existing between centres and their different level of complexity. However, we consider that these can represent a good reflection of what happens in the hospital setting. On the other hand, we have no data on efficacy, toxicity or costs, because these were not the main objectives of the study, and mostly because there was no clinical follow-up report at the cut-off year for the study. It seems complicated to conduct an optimal traceability of the outcomes of the use of off-label medications, and in most publications there is little or incomplete information. It would be desirable to have good information available on clinical follow-up, and to include this aspect in subsequent studies. The volume of applications for off-label use during one year in this centre can be considered important and superior to that detected in other studies9,15,20,21. Even so, it is likely that figures were underestimated if some professionals were not aware of the off-label nature of some prescriptions and did not present the relevant application, maybe because this was already part of their usual clinical practice, or also because this period was still very close to the administrative changes enforced by the RD 1015/2009.
The high use of immunosuppressants is probably associated with the wide range of diseases with an autoimmune basis in common, leading to their request for different dermatological and ophthalmological indications, as well as for vasculitis, sarcoidosis or SLE, among others. In the case of lupus, different biologic drugs have been used off-label; however, none of them has demonstrated efficacy in good-quality clinical trials. This can be partly understood by the difficulty represented by the heterogeneous nature of its clinical presentation22. In our opinion, the reason to prescribe some of these medications in non-authorized indications can be associated with an assumed class effect, or by extension to indications associated in their physiological basis, or even for processes that have some symptoms in common. The medication with the highest number of applications was precisely the immunosuppressant tacrolimus, basically in terms of its use for preventing rejection in lung transplant, an indication for which the Hospital Reina Sofía is a centre of reference.
The off-label use of antineoplastic drugs can be understood if it is taken into account that Oncohaematology is one of the areas with higher therapeutic needs. There are published data estimating that the third part of oncological patients will receive at least one off-label antineoplastic treatment, and that 27% of these are prescribed for indications different to those approved23.
This profile, with a special prominence by immunosuppressants and biologic therapy, is similar to the one in recent publications20,21, but very different to the one found in the first studies conducted on off-label uses, where the therapeutic groups for cardiovascular and nervous systems (mostly gabapentin and amitriptyline) were the most associated with these prescriptions1; however, it should be taken into account that these initial studies were conducted with prescriptions by GPs in the community, and not by specialists within the hospital setting.
When drugs are compared individually and not by groups, it is evident that there is a very wide variability in this type of prescriptions. In fact, the most requested medications in our study were tacrolimus, mycophenolate, colistimethate and everolimus, while in a relevant study conducted in various centres, the most widely used drugs were rituximab, botulinum toxin, omalizumab and anakinra9. And there are more differences, because in some cases there is not even coincidence in the indications for the same medication; for example in the case of tacrolimus (requested for chronic urticaria in this Catalan study9, and for lung transplant, SLE and membranous nephropathy in the Reina Sofía), or ustekinumab (for Crohn’s Disease and refractory atopic dermatitis, respectively). There are more recent data from a wide series covering a 5-year period, where the most requested drugs were rituximab and bevacizumab24.
In any case, it is important to point out that many of these drugs have been recently marketed, and they are sophisticated, expensive, and present a higher complexity of use. This requires a careful evaluation of the benefit/ risk/cost balance, particularly for new biologics25.
Regarding the scientific support for all these indications, there was a low level of evidence in almost half of the cases (47.6%), and these figures were not very different to those of similar studies (51.8%)9. It is true that there is no complete homogeneity at the time of classifying the levels of evidence into high and low; in our case, in agreement with other publications1,14,15, it was simplified by defining as good or acceptable level of evidence any use that had at least one clinical trial at the time of application. According to a Canadian study conducted at Primary Care level, apparently those physicians with training oriented by evidence-based medicine are less likely to prescribe off-label14. But it is quite questionable that this outcome can be extrapolated to the hospital setting, where clinical scenarios and, most of all, therapeutic requirements are completely different.
It seemed to us that learning about the changes in evidence levels, and any potential improvements in the support for these indications over time, would be an indirect way to assess the scientific activity in these areas, which occurred in approximately the third part of off-label uses. At the same time, looking for clinical trial setup could also show some research dynamism, which was confirmed in 67% of uses, with the highest number of clinical trials registered in oncological diseases such as ovarian cancer, glioblastoma, mantle cell lymphoma, and melanoma.
Finally, the extension of the use as new indication only occurred in 11% of cases. This might seem a low proportion, but there is no other study that has explored this aspect in order to compare it. We could interpret the authorization of these variations in the product specifications as the finalization of a process where evidence has been summing up in order to confirm said use. In fact, there is a very good correlation between the indications approved and the high levels of evidence that these uses had at the time of application. However, there are off-label uses supported by good- quality studies that have not achieved an extended indication (for example, mycophenolate for lupus nephritis, tacrolimus for lung transplant, and botulinum toxin for achalasia). This fact could be associated with certain dissociation between the methodological quality of the studies and certain questionable or non-conclusive efficacy outcomes, but also with another series of factors, including the requirements by healthcare authorities in order to authorize the new indication, or the lack of interest by pharmaceutical companies to request it.
Given their frequency and importance, off-label uses are a major area of clinical practice, where the gaps in evidence should lead to a higher reflection about a series of signals like: recent drugs, new off-label uses, medications with major adverse reactions, and high-cost medications26. And their use should be guided using the principles and support of evidence-based medicine27. However, a series of advantages derived of these uses could be acknowledged, such as the innovative nature in clinical practice, the access to therapies with emerging evidence, and the likelihood to treat certain orphan diseases28. In this sense, according to the Declaration of Helsinki, the need to treat will prevail, and “the physician, with the informed consent by the patient, can be allowed to use non-confirmed interventions if, according to their judgment, these will offer some hope of saving the patient’s life, restore their health, or alleviate their suffering”29.
But, on the other hand, these uses will have obvious consequences in pharmacy policy and healthcare management, if we take into account the costs as well as the safety profile of those medications detected with the highest off-label use. Therefore, a good collaboration is required between healthcare administrations and centres, with the aim to guarantee their optimal use. Thus, different Autonomous Communities are establishing their own regulations30, including matters such as the creation of Technical Committees for the evaluation of certain medications to be used in special situations, when these have a high healthcare or economic impact. At hospital level, Pharmacy and Therapeutics Committees are responsible for assessing the applications for medications in special situations. But it is a fact that there are differences between the committees in different centres, both in management as in the criteria to be applied for the evaluations, and this could lead to differences in the possibilities to access specific treatments. It is also apparent that the quality of evidence has not demonstrated being a decisive variable for the approval of use of these treatments; however, there seems to be a clear influence by patient age and pharmaceutical cost24. This can be understood if we incorporate to this scenario the existence of alternative treatments with a similar efficacy-safety profile but a lower cost. Therefore, this is a matter of conciliating the assessment of aspects such as the level of evidence for the suggested off-label use, cost, existence of valid alternative options, patient characteristics, and the severity or urgency of the process.
An important volume of off-label uses without good evidence has been detected, and this identifies these indications and medications as interesting lines of research, but requiring follow-up for effectiveness and costs. Special attention should be paid to the immunosuppressant group, not only due to the high number of applications presented, but also to the variety of indications for which these drugs are prescribed.











 text in
text in