Introduction
Type 2 Diabetes Mellitus (T2DM) is a chronic disease associated at long term with micro and macrovascular complications which involve multiple organs; its prevalence has been increasing during the past decades. It is currently estimated that in Spain there is a 13.8% overall prevalence of Diabetes Mellitus (Type 1 and Type 2) in >18-year-old persons1; TDM22 is more frequent (up to 90% of cases). Poor metabolic control is associated with an increase in complications and premature death, and it is also the first cause for blindness, renal replacement treatment (dialysis/transplant) and non-traumatic amputation in Western countries. An early and multifactorial treatment will delay the development of complications, and improve quality of life and life expectancy3.
There is a multidisciplinary therapeutic approach for the disease, including both pharmacological and non-pharmacological strategies as measures for preventing comorbidities and long-time complications. The first step will generally consist in initiating treatment with metformin, added on to diet and exercise4.
Canagliflozin is a new antidiabetic treatment within the group of reversible inhibitors of sodium-glucose cotransporter 2 (SGLT-2). Currently it is marketed alone or associated with metformin. It is indicated as monotherapy for adults with T2DM, when diet and exercise alone cannot achieve enough glycaemic control in patients for whom the use of metformin has been considered inadequate due to lack of tolerability or contraindications. It is also indicated as complementary treatment administered with other antidiabetic treatments, such as insulin or metformin, when these cannot achieve an adequate glycaemic control in association with diet and exercise5.
Canagliflozin is a recently-marketed medication (April, 2015), and therefore it is subject to additional follow-up (inverted black triangle). Being a new molecule approved for the first time, there is limited information on safety, and only information from clinical trials is available6.
Regarding the safety profile of canagliflozin, it was observed in the CANVAS study7 that there was an increase in the incidence of non-traumatic amputation of lower limbs in patients treated with canagliflozin vs. patients treated with placebo; similar outcomes were obtained in the CANVAS-R study8. Due to these results, the Spanish Agency of Medicines and Health Products (AEMPS) issued a Safety Information Note (May, 2016) about canagliflozin regarding patients with risk of amputations9.
The objective of this study is to analyze the impact of a strategy for canagliflozin adequacy after recommendations of use were issued, based on the Pharmacovigilance (PV) Notes issued by the AEMPS, as well as to assess the level of acceptance by physicians.
Methods
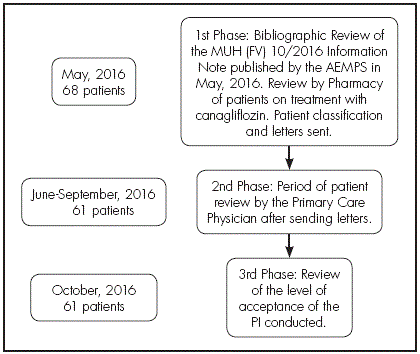
A prospective study conducted from May, 2016 to October, 216, based on an intervention strategy linked to the issuance of an Information Note by the AEMPS on canagliflozin, in the South-Seville Health Management Area, with a population of 406,701 patients, including a Hospital with all specialties and 30 health centres. The intervention strategy was conducted by the Pharmacy Unit of the Area, and was divided into three phases.
In the first phase, after the MUH (FV) Information Note 10/2016 was published on May, 2016 by the AEMPS, a bibliographic review was conducted on the original sources motivating said note7-8.
A review was conducted on those patients who were on treatment by electronic prescription with canagliflozin alone or associated with metformin, from January to March, 2016. After this review, patients were classified into three groups:
Those patients who presented risk factors for amputation that could be subject to monitoring: previous amputations, peripheral vascular disease, or pre-existing neuropathy.
Patients with factors that might lead to treatment interruption, defined as important complications in lower limbs such as skin ulcers, osteomyelitis or gangrenes10.
The rest of patients on treatment with canagliflozin; watching would be recommended, with the aim to avoid the previously mentioned risk factors, and in order to detect signs and symptoms caused by the depletion of body water and salts.
Three types of information letters were prepared according to the group where each patient was included; these were sent to Primary Care Physicians.
Letter 1. Stating the need for patient monitoring.
Letter 2. Recommending treatment interruption.
Letter 3. Recommending the need to watch patients.
The Information Note MUH (FV) 10/2016 was e-mailed to the managers of the Clinical Management Unit of Primary Care, to be distributed to all physicians; different letters were also attached to be sent to those physicians with patients who had been prescribed this treatment, with the indication of the type of intervention to be conducted according to the Pharmacy Unit.
In a second phase, Primary Care Physicians reviewed the different patients indicated by Pharmacy through the letters sent, from June to September.
Finally, in a third phase conducted four months after these letters were sent (October, 2016), there was a review of the level of acceptance of the intervention conducted (Figure 1).
The variables analyzed in the study were: demographical data, treatment with canagliflozin alone or in combination with metformin, mean dose, risk factors for amputation (previous amputations, peripheral vascular disease, or pre-existing neuropathy), major complications in lower limbs such as skin ulcers, osteomyelitis or gangrene, and level of acceptance of the intervention (accepted and interruption of treatment with the drug, acceptance and patient monitoring, acceptance and watching the patients, or no relevant action taken by the physician).
Patient selection was conducted through the MicroStrategy® database for prescription invoicing by the Andalusian Health Service. This program collects data derived of the prescription and dispensing of medications and health products by different healthcare professionals. Data collection was conducted by consulting the DIRAYA-AP® electronic clinical record and the Prescripciones 5® computer system for electronic prescription. The IBM SPSS Statistics® 20.0 program for Windows (IBM Corp., Armonk, NY) was used for data analysis. There was a descriptive analysis through mean and standard deviation, or median and interquartile range (in case of asymmetry) for quantitative variables, and through frequencies and percentages for qualitative variables.
Results
In total, sixty-eight (68) patients on treatment with canagliflozin were included at the start of the study. During the stage of patient identification, seven patients were excluded due to interruption of treatment with the drug at the time of the study or patient’s exitus; therefore, treatment adequacy was evaluated in a total population of 61 patients. The mean age of the 61 patients reviewed by Pharmacy was 62.3 years (SD: 11.3); 54.1% of patients were male.
The mean dose of canagliflozin was 89.3 mg (SD: 20.7), and the mean duration of treatment by the time of the study was 4.7 months (SD: 2.9). In total, 59.0% patients were on treatment with canagliflozin alone, the rest had been prescribed a combination of canagliflozin with metformin.
Risk factors for monitoring were present in six patients: four patients presented vascular conditions, one suffered neuropathy, and another one presented vascular disease and previous amputation.
Only one patient presented risk factors for drug interruption, after suffering previous amputations and infections associated with the development of skin ulcers. The rest of patients (n=54) had no risk factors.
After Pharmacy Intervention (PI) (Table 1), treatment was closely watched in one of the 6 (9.8%) patients pending monitoring, regarding hydration status and the potential development of other risk factors and complications. Treatment was interrupted in one patient, another patient was monitored, and no intervention was conducted by the PCP in three patients. Therefore, the intervention proposed was only conducted in one single patient.
Table 1 Type of action taken by the Primary Care Physician after the PI conducted through sending different letter models

There was an effective PI in the case of the patient with risk factors for treatment interruption, because treatment was interrupted.
Of the rest of patients (88.5 %; n=54) for whom a letter was sent to watch their treatment and risk factors, 29.6% (n=16) had their treatment monitored, 14.8% (n=8) had their treatment interrupted, and 3.7% (n=2) were closely watched. In the rest of patients (51.8%; n=28) no clinical interventions were conducted after the indications by the Pharmacy Unit.
Only 6.6% of PIs were conducted by the physician adequately, as indicated.
Overall, the outcomes obtained regardless of the type of intervention proposed through the letter were: action was taken for 49.2% (n=30) of patients, (56.7% were monitored, treatment was interrupted for 33.3%, and 10.0% were closely watched). No action was taken for 50.8% of patients.
Discussion
This study shows that the strategies targeted to a prompt adequacy of treatment to FV alerts have been effective, with a high level of acceptance by Primary Care Physicians. However, the type of intervention proposed through the letter was not as expected: in some cases, PCPs adopted other measures. This could be because the physician found evidence to adopt this measure instead of the one proposed by the Pharmacy Unit, when re-evaluating the treatment and clinical record of the patient,
There are many studies showing that different interventions by the Pharmacist for a higher adequacy of antidiabetic treatment allow to obtain better health outcomes11-13.
In our setting, there are no studies on the level of acceptance of PIs after PV alerts for drugs associated with diabetes; however, there are other studies such as the one conducted by Cantudo-Cuenca et al., about interventions on strontium ranelate after FV alerts in patients with osteoporosis, which shows a high degree of acceptance of these PIs, higher than the one found in our study; however, their study could be biased because the population was larger than the one in our research14.
Regarding studies about PIs in the setting of diabetes, Vélez-Díaz-Pallarés et al. showed similar results regarding the degree of acceptance in patients with renal impairment treated with metformin15.
We must highlight the limitation of a reduced study population. This can be explained by the fact that canagliflozin is a recently marketed medication (April, 2015), and is subject to additional follow-up due to safety reasons.
Another study limitation can be due to the fact that PCPs were not involved in its design, and this originated different communication problems, such as the low distribution of the Information Note and the letters by CMU managers to physicians indicating patients on treatment with this drug. This shows the need to establish effective ways of communication between pharmacists and clinicians.
On the other hand, we have found the limitation of a low level of information recorded in patient clinical records. This might have led to the difference between the proposed PI and the intervention finally conducted by the PCP, who was aware of the real clinical situation of the patient, not accurately shown in their clinical record.
Taking into account the low population in our study, multicentre studies could be proposed for a higher statistical power. Besides, intervention studies could be conducted for all SGLT inhibitors.
Summing up, PIs improve the safety of diabetic patients treated with canagliflozin, which contributes to a reduction in the risk of amputations. There has been good acceptance of PIs, though physicians did not conduct the best intervention according to what had been recommended. Due to the outcomes obtained, it should be proposed to send new letters to each of those patients on treatment with this drug, in order to conduct a new review. Besides, it would be advisable to implement complementary actions in order to improve the outcomes in those patients selected for closer monitoring, as well as systems for direct communication between physicians and pharmacists, and improving record systems.
Contribution to scientific literature
The CANVAS study has detected that canagliflozin increases the risk of amputations. Currently it is unknown if there is any class effect, and therefore it cannot be ruled out that said risk might be extended to other SGLT-2 inhibitors.
The Spanish Agency of Medicines and Health Products has issued a Pharmacovigilance Information Note on canagliflozin.











 text in
text in